Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database
- PMID: 34706874
- PMCID: PMC8862028
- DOI: 10.1136/annrheumdis-2021-221276
Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database
Abstract
Objective: To report long-term safety from the completed extension trial of baricitinib, an oral selective Janus kinase inhibitor, in patients with active rheumatoid arthritis (RA).
Methods: Treatment-emergent adverse events are summarised from an integrated database (9 phase III/II/Ib and 1 long-term extension) of patients who received any baricitinib dose (All-bari-RA). Standardised incidence ratio (SIR) for malignancy (excluding non-melanoma skin cancer (NMSC)) and standardised mortality ratio (SMR) were estimated. Additional analysis was done in a subset of patients who had ever taken 2 mg or 4 mg baricitinib.
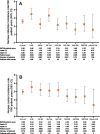
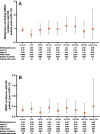
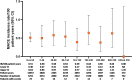
Results: 3770 patients received baricitinib (14 744 patient-years of exposure (PYE)). All-bari-RA incidence rates (IRs) per 100 patient-years at risk were 2.6, 3.0 and 0.5 for serious infections, herpes zoster and major adverse cardiovascular events (MACE), respectively. In patients aged ≥50 with ≥1 cardiovascular risk factor, the IR for MACE was 0.77 (95% CI 0.56 to 1.04). The IR for malignancy (excluding NMSC) during the first 48 weeks was 0.6 and remained stable thereafter (IR 1.0). The SIR for malignancies excluding NMSC was 1.07 (95% CI 0.90 to 1.26) and the SMR was 0.74 (95% CI 0.59 to 0.92). All-bari-RA IRs for deep vein thrombosis (DVT)/pulmonary embolism (PE), DVT and PE were 0.5 (95% CI 0.38 to 0.61), 0.4 (95% CI 0.26 to 0.45) and 0.3 (95% CI 0.18 to 0.35), respectively. No clear dose differences were noted for exposure-adjusted IRs (per 100 PYE) for deaths, serious infections, DVT/PE and MACE.
Conclusions: In this integrated analysis including long-term data of baricitinib from 3770 patients (median 4.6 years, up to 9.3 years) with active RA, baricitinib maintained a similar safety profile to earlier analyses. No new safety signals were identified.
Trial registration number: NCT01185353, NCT00902486, NCT01469013, NCT01710358, NCT02265705, NCT01721044, NCT01721057, NCT01711359 and NCT01885078.
Keywords: autoimmune diseases; biological therapy; rheumatoid arthritis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PCT has received consultant fees from AbbVie, Biogen, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, BMS, Pfizer, Roche, Celltrion, Sanofi, Nordic Pharma, Fresenius and UCB; and grant/research support from Celgene, Galapagos, Janssen and Lilly. TT has received grant/research support from Daiichi Sankyo, Takeda, Nippon Kayaku, JCR Pharma, Astellas, Chugai, AbbVie GK, Asahi Kasei Pharma, Mitsubishi Tanabe, UCB Japan and Eisai; consulting fees from Astellas, AbbVie GK, Gilead, Daiichi Sankyo, Taisho Pharma, Nippon Kayaku, Bristol-Myers Squibb, GlaxoSmithKline, Eli Lilly, Novartis, Mitsubishi Tanabe and Chugai; and speakers’ bureau fees from Mitsubishi Tanabe, Pfizer, Astellas, Daiichi Sankyo, Eisai, Sanofi, Novartis, Eli Lilly, Gilead, AbbVie GK, Bristol-Myers Squibb and Chugai. G-RRB has received honoraria for lectures and consulting from AbbVie, Gilead, Lilly and Pfizer. PD has received fees for speakers' bureau from BMS, Sanofi, Eli Lilly and Celltrion. JSS has received grant/research support from AbbVie, Eli Lilly and Company, Janssen, MSD, Novartis, Pfizer and Roche; and consultancy fees from AbbVie, Amgen, AstraZeneca, Astro, BMS, Celgene, Celltrion, Chugai, Eli Lilly and Company, Gilead, ILTOO, Janssen, MedImmune, MSD, Novartis, Pfizer, Roche, Samsung, Sanofi and UCB. WD, MI, JRT and NB are employees and stockholders of Eli Lilly and Company. KLW has received grant/research support from Pfizer and BMS; and consulting fees from Pfizer, UCB, AbbVie, Eli Lilly and Company, Amgen, BMS, Galapagos and Gilead.
Figures