Testing the effects of combining azithromycin with inhaled tobramycin for P. aeruginosa in cystic fibrosis: a randomised, controlled clinical trial
- PMID: 34706982
- PMCID: PMC9043040
- DOI: 10.1136/thoraxjnl-2021-217782
Testing the effects of combining azithromycin with inhaled tobramycin for P. aeruginosa in cystic fibrosis: a randomised, controlled clinical trial
Abstract
Rationale: Inhaled tobramycin and oral azithromycin are common chronic therapies in people with cystic fibrosis and Pseudomonas aeruginosa airway infection. Some studies have shown that azithromycin can reduce the ability of tobramycin to kill P. aeruginosa. This trial was done to test the effects of combining azithromycin with inhaled tobramycin on clinical and microbiological outcomes in people already using inhaled tobramycin. We theorised that those randomised to placebo (no azithromycin) would have greater improvement in forced expiratory volume in one second (FEV1) and greater reduction in P. aeruginosa sputum in response to tobramycin.
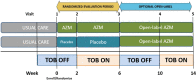
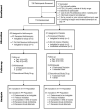
Methods: A 6-week prospective, randomised, placebo-controlled, double-blind trial testing oral azithromycin versus placebo combined with clinically prescribed inhaled tobramycin in individuals with cystic fibrosis and P. aeruginosa airway infection.
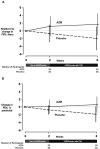
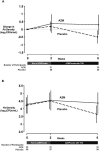
Results: Over a 6-week period, including 4 weeks of inhaled tobramycin, the relative change in FEV1 did not statistically significantly differ between groups (azithromycin (n=56) minus placebo (n=52) difference: 3.44%; 95% CI: -0.48 to 7.35; p=0.085). Differences in secondary clinical outcomes, including patient-reported symptom scores, weight and need for additional antibiotics, did not significantly differ. Among the 29 azithromycin and 35 placebo participants providing paired sputum samples, the 6-week change in P. aeruginosa density differed in favour of the placebo group (difference: 0.75 log10 CFU/mL; 95% CI: 0.03 to 1.47; p=0.043).
Conclusions: Despite having greater reduction in P. aeruginosa density in participants able to provide sputum samples, participants randomised to placebo with inhaled tobramycin did not experience significantly greater improvements in lung function or other clinical outcomes compared with those randomised to azithromycin with tobramycin.
Keywords: bacterial Infection; cystic fibrosis; respiratory Infection.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DN and NM-H, as part of their roles at the Cystic Fibrosis (CF) Foundation Therapeutics Development Network Coordinating Centre, provide consulting to industry sponsors who are developing new drug therapies for cystic fibrosis. Some of these sponsors are working to develop antimicrobial agents. PS and DN report grants from Vertex and Gilead Sciences outside of the published work. RG and GR-B report grants from Vertex outside of the published work. LS reports grants from Merck Co and Bill and Melinda Gates Foundation outside of the published work and payments for Data Safety Monitoring Board or Advisory Boards from Merck Co. Multiple authors have grant support or payments from the CF Foundation.
Figures

References
-
- 2019 cystic fibrosis Foundation patient registry annual data report 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
