A synthetic antibiotic class overcoming bacterial multidrug resistance
- PMID: 34707295
- PMCID: PMC8549432
- DOI: 10.1038/s41586-021-04045-6
A synthetic antibiotic class overcoming bacterial multidrug resistance
Abstract
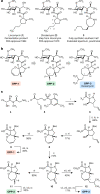
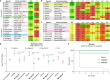
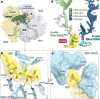
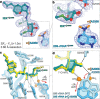
The dearth of new medicines effective against antibiotic-resistant bacteria presents a growing global public health concern1. For more than five decades, the search for new antibiotics has relied heavily on the chemical modification of natural products (semisynthesis), a method ill-equipped to combat rapidly evolving resistance threats. Semisynthetic modifications are typically of limited scope within polyfunctional antibiotics, usually increase molecular weight, and seldom permit modifications of the underlying scaffold. When properly designed, fully synthetic routes can easily address these shortcomings2. Here we report the structure-guided design and component-based synthesis of a rigid oxepanoproline scaffold which, when linked to the aminooctose residue of clindamycin, produces an antibiotic of exceptional potency and spectrum of activity, which we name iboxamycin. Iboxamycin is effective against ESKAPE pathogens including strains expressing Erm and Cfr ribosomal RNA methyltransferase enzymes, products of genes that confer resistance to all clinically relevant antibiotics targeting the large ribosomal subunit, namely macrolides, lincosamides, phenicols, oxazolidinones, pleuromutilins and streptogramins. X-ray crystallographic studies of iboxamycin in complex with the native bacterial ribosome, as well as with the Erm-methylated ribosome, uncover the structural basis for this enhanced activity, including a displacement of the [Formula: see text] nucleotide upon antibiotic binding. Iboxamycin is orally bioavailable, safe and effective in treating both Gram-positive and Gram-negative bacterial infections in mice, attesting to the capacity for chemical synthesis to provide new antibiotics in an era of increasing resistance.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
A.G.M., M.J.M., K.J.S., J.D.M. and G.T are inventors in a provisional patent application submitted by the President and Fellows of Harvard College covering antibiotics of the type described in this work. A.G.M., M.J.M. and K.J.S. have filed an international patent application WO/2019/032936 ‘Lincosamide Antibiotics and Uses Thereof’. A.G.M. and M.J.M. have filed an international patent application WO/2019/032956 ‘Lincosamide Antibiotics and Uses Thereof’.
Figures



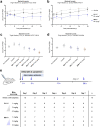

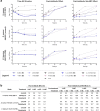
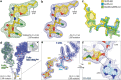
Comment in
-
Synthetic antibiotic fights resistance.Nat Rev Drug Discov. 2022 Jan;21(1):20. doi: 10.1038/d41573-021-00191-8. Nat Rev Drug Discov. 2022. PMID: 34754106 No abstract available.
References
-
- Antibiotic Resistance Threats in the United States, 2019 (Department of Health and Human Services, CDC, 2019); www.cdc.gov/DrugResistance/Biggest-Threats html.
-
- Scientific Roadmap for Antibiotic Discovery (Pew Charitable Trusts, 2016); http://www.pewtrusts.org/antibiotic-discovery.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

