Parkinson's Disease Dementia: Synergistic Effects of Alpha-Synuclein, Tau, Beta-Amyloid, and Iron
- PMID: 34707492
- PMCID: PMC8542689
- DOI: 10.3389/fnagi.2021.743754
Parkinson's Disease Dementia: Synergistic Effects of Alpha-Synuclein, Tau, Beta-Amyloid, and Iron
Abstract
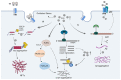
Parkinson's disease dementia (PDD) is a common complication of Parkinson's disease that seriously affects patients' health and quality of life. At present, the process and pathological mechanisms of PDD remain controversial, which hinders the development of treatments. An increasing number of clinical studies have shown that alpha-synuclein (α-syn), tau, beta-amyloid (Aβ), and iron are closely associated with PDD severity. Thus, we inferred the vicious cycle that causes oxidative stress (OS), due to the synergistic effects of α-syn, tau, Aβ, and, iron, and which plays a pivotal role in the mechanism underlying PDD. First, iron-mediated reactive oxygen species (ROS) production can lead to neuronal protein accumulation (e.g., α-syn andAβ) and cytotoxicity. In addition, regulation of post-translational modification of α-syn by iron affects the aggregation or oligomer formation of α-syn. Iron promotes tau aggregation and neurofibrillary tangles (NFTs) formation. High levels of iron, α-syn, Aβ, tau, and NFTs can cause severe OS and neuroinflammation, which lead to cell death. Then, the increasing formation of α-syn, Aβ, and NFTs further increase iron levels, which promotes the spread of α-syn and Aβ in the central and peripheral nervous systems. Finally, iron-induced neurotoxicity promotes the activation of glycogen synthase kinase 3β (GSK3β) related pathways in the synaptic terminals, which in turn play an important role in the pathological synergistic effects of α-syn, tau and Aβ. Thus, as the central factor regulating this vicious cycle, GSK3β is a potential target for the prevention and treatment of PDD; this is worthy of future study.
Keywords: Parkinson’s disease dementia; alpha-synuclein; beta-amyloid; iron; tau.
Copyright © 2021 Han, Fan, Wu, Huang, Li, Zhao, Ji and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., et al. . (2006). Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic lewy body disease. J. Biol. Chem. 281, 29739–29752. 10.1074/jbc.M600933200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

