Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis
- PMID: 34707602
- PMCID: PMC8542872
- DOI: 10.3389/fimmu.2021.714170
Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis
Abstract
There is a significant research gap in meta-analysis on the efficacy and safety of coronavirus disease 2019 (COVID-19) vaccines. This study analyzed the efficacy of COVID-19 vaccines. Published phase I, phase II, and phase III trials analyzing safety and immunogenicity and phase III randomized clinical trials evaluating the efficacy of COVID-19 vaccines were included. We searched MEDLINE, Scopus, and The Lancet for published articles evaluating the relative reduction in COVID-19 risk after vaccination. Selected literatures were published between December 15, 2019 and May 15, 2021 on the safety, efficacy, and immunogenicity of COVID-19 vaccines. This meta-analysis included studies that confirmed cases of COVID-19 using reverse transcriptase polymerase chain reaction. This study detected 8,926 eligible research articles published on COVID-19 vaccines. Of these, 25 studies fulfilled the inclusion criteria. Among the selected articles, 19 randomized clinical trials, 2 non-randomized clinical trials, and 3 observational studies were analyzed. Seven (28%) studies were included in the meta-analysis. The efficacy of the adenovirus vector vaccine was 73% (95% CI = 69-77) and that of the messenger RNA (mRNA) vaccine was 85% (95% CI = 82-88) in participants aged ≥18 years. There are no reports of clinical trials in participants aged under 16 years. The production of neutralizing antibodies against receptor-binding domains (RBDs) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in >90% of the vaccinated samples was reported within 0-30 days of the first or the second dose of the vaccine. Pain at the injection site was the most common local symptom in people receiving mRNA vaccines (29%-85% of participants). Fever (0.2%-95%) was the most prevalent in people receiving adenovirus vector vaccines, and fatigue (8.4%-55%) was the most common side effect in people receiving the mRNA vaccines. Studies suggest that mRNA vaccines and adenovirus vector vaccines can provide moderate to high protection against COVID-19 infection in people over 18 years. Evidence of the long-term protection of the vaccines in people aged under 16 years against the multiple variants of COVID-19 are limited. This study will provide an integrated evaluation on the efficacy, safety, and immunogenicity of the COVID-19 vaccines.
Keywords: COVID-19 vaccines; efficacy; immunogenicity; meta-analysis; safety.
Copyright © 2021 Sharif, Alzahrani, Ahmed and Dey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
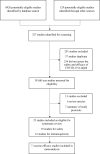

References
-
- COVID-19 Map - Johns Hopkins Coronavirus Resource Center. (2021). Available at: https://coronavirus.jhu.edu/map.html (Accessed May 15, 2021).
-
- World Health Organization . WHO Coronavirus Disease (COVID-19) Dashboard. (2021). Available at: https://covid19.who.int (Accessed May 15, 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

