Characterization of the Infant Immune System and the Influence and Immunogenicity of BCG Vaccination in Infant and Adult Rhesus Macaques
- PMID: 34707617
- PMCID: PMC8542880
- DOI: 10.3389/fimmu.2021.754589
Characterization of the Infant Immune System and the Influence and Immunogenicity of BCG Vaccination in Infant and Adult Rhesus Macaques
Abstract
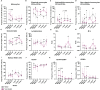
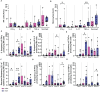
In many countries where tuberculosis (TB) is endemic, the Bacillus Calmette-Guérin (BCG) vaccine is given as close to birth as possible to protect infants and children from severe forms of TB. However, BCG has variable efficacy and is not as effective against adult pulmonary TB. At present, most animal models used to study novel TB vaccine candidates rely on the use of adult animals. Human studies show that the infant immune system is different to that of an adult. Understanding how the phenotypic profile and functional ability of the immature host immune system compares to that of a mature adult, together with the subsequent BCG immune response, is critical to ensuring that new TB vaccines are tested in the most appropriate models. BCG-specific immune responses were detected in macaques vaccinated within a week of birth from six weeks after immunization indicating that neonatal macaques are able to generate a functional cellular response to the vaccine. However, the responses measured were significantly lower than those typically observed following BCG vaccination in adult rhesus macaques and infant profiles were skewed towards the activation and attraction of macrophages and monocytes and the synthesis in addition to release of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α. The frequency of specific immune cell populations changed significantly through the first three years of life as the infants developed into young adult macaques. Notably, the CD4:CD8 ratio significantly declined as the macaques aged due to a significant decrease in the proportion of CD4+ T-cells relative to a significant increase in CD8+ T-cells. Also, the frequency of both CD4+ and CD8+ T-cells expressing the memory marker CD95, and memory subset populations including effector memory, central memory and stem cell memory, increased significantly as animals matured. Infant macaques, vaccinated with BCG within a week of birth, possessed a significantly higher frequency of CD14+ classical monocytes and granulocytes which remained different throughout the first three years of life compared to unvaccinated age matched animals. These findings, along with the increase in monokines following vaccination in infants, may provide an insight into the mechanism by which vaccination with BCG is able to provide non-specific immunity against non-mycobacterial organisms.
Keywords: BCG; age comparison; immunology; infant; infant vaccination; macaque.
Copyright © 2021 Sarfas, White, Sibley, Morrison, Gullick, Lawrence, Dennis, Marsh, Fletcher and Sharpe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Immune cell population dynamics following neonatal BCG vaccination and aerosol BCG revaccination in rhesus macaques.Sci Rep. 2024 Jul 23;14(1):16993. doi: 10.1038/s41598-024-67861-6. Sci Rep. 2024. PMID: 39043848 Free PMC article.
-
Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations.Tuberculosis (Edinb). 2016 Dec;101:174-190. doi: 10.1016/j.tube.2016.09.004. Epub 2016 Oct 8. Tuberculosis (Edinb). 2016. PMID: 27865390 Free PMC article.
-
Nanocages engineered from Bacillus Calmette-Guerin facilitate protective Vγ2Vδ2 T cell immunity against Mycobacterium tuberculosis infection.J Nanobiotechnology. 2022 Jan 15;20(1):36. doi: 10.1186/s12951-021-01234-3. J Nanobiotechnology. 2022. PMID: 35033108 Free PMC article.
-
Is interferon-gamma the right marker for bacille Calmette-Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology.Clin Exp Immunol. 2012 Sep;169(3):213-9. doi: 10.1111/j.1365-2249.2012.04614.x. Clin Exp Immunol. 2012. PMID: 22861360 Free PMC article. Review.
-
Neonatal and infant immunity for tuberculosis vaccine development: importance of age-matched animal models.Dis Model Mech. 2020 Sep 15;13(9):dmm045740. doi: 10.1242/dmm.045740. Dis Model Mech. 2020. PMID: 32988990 Free PMC article. Review.
Cited by
-
Immune cell population dynamics following neonatal BCG vaccination and aerosol BCG revaccination in rhesus macaques.Sci Rep. 2024 Jul 23;14(1):16993. doi: 10.1038/s41598-024-67861-6. Sci Rep. 2024. PMID: 39043848 Free PMC article.
-
Immune mechanisms mediating the heterologous effects of BCG vaccination: a systematic review.Front Immunol. 2025 May 19;16:1567111. doi: 10.3389/fimmu.2025.1567111. eCollection 2025. Front Immunol. 2025. PMID: 40458396 Free PMC article.
-
Systematic review of innate immune responses against Mycobacterium tuberculosis complex infection in animal models.Front Immunol. 2025 Jan 30;15:1467016. doi: 10.3389/fimmu.2024.1467016. eCollection 2024. Front Immunol. 2025. PMID: 39949719 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

