GU-CA-COVID: a clinical audit among Italian genitourinary oncologists during the first COVID-19 outbreak
- PMID: 34707691
- PMCID: PMC8543560
- DOI: 10.1177/17562872211054302
GU-CA-COVID: a clinical audit among Italian genitourinary oncologists during the first COVID-19 outbreak
Erratum in
-
Corrigendum to "GU-CA-COVID: a clinical audit among Italian genitourinary oncologists during the first COVID-19 outbreak".Ther Adv Urol. 2021 Dec 6;13:17562872211067689. doi: 10.1177/17562872211067689. eCollection 2021 Jan-Dec. Ther Adv Urol. 2021. PMID: 34899989 Free PMC article.
Abstract
Background: Considering the growing genitourinary (GU) cancer population undergoing systemic treatment with immune checkpoint inhibitors (ICIs) in the context of the COVID-19 pandemic, we planned a clinical audit in 24 Italian institutions treating GU malignancies.
Objective: The primary objective was investigating the clinical impact of COVID-19 in GU cancer patients undergoing ICI-based therapy during the first outbreak of SARS-CoV-2 contagion in Italy.
Design setting and participants: The included centers were 24 Oncology Departments. Two online forms were completed by the responsible Oncology Consultants, respectively, for metastatic renal cell carcinoma (mRCC) and metastatic urothelial carcinoma (mUC) patients receiving at least one administration of ICIs between 31 January 2020 and 30 June 2020.
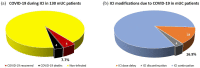
Results and limitation: In total, 287 mRCC patients and 130 mUC patients were included. The COVID-19 incidence was, respectively, 3.5%, with mortality 1%, in mRCC patients and 7.7%, with mortality 3.1%, in mUC patients. In both groups, 40% of patients developing COVID-19 permanently discontinued anticancer treatment. The pre-test SARS-CoV-2 probability in the subgroup of patients who underwent nasal/pharyngeal swab ranged from 14% in mRCC to 26% in mUC. The main limitation of the work was its nature of audit: data were not recorded at the single-patient level.
Conclusion: GU cancer patients undergoing active treatment with ICIs have meaningful risk factors for developing severe events from COVID-19 and permanent discontinuation of therapy after the infection. Treatment delays due to organizational issues during the pandemic were unlikely to affect the treatment outcome in this population.
Keywords: COVID-19; SARS-CoV-2; bladder cancer; cancer patients; genitourinary cancers; renal cancer: urothelial cancer.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that there is no conflict of interest for the present article, except those reported herein: Melissa Bersanelli received honoraria as a speaker at scientific events by Bristol-Myers Squibb (BMS), Novartis, AstraZeneca, Pierre Fabre, and Pfizer, and as a consultant for advisory role by Novartis, BMS, IPSEN, and Pfizer; she also received fees for copyright transfer by Sciclone Pharmaceuticals and research funding by Roche S.p.A., Seqirus UK, Pfizer, Novartis, BMS, AstraZeneca, and Sanofi Genzyme. Sebastiano Buti received honoraria as a speaker at scientific events and advisory role by BMS, Pfizer, Merck Sharp & Dohme (MSD), Ipsen, Roche, Eli-Lilly, AstraZeneca, and Novartis; he also received research funding from Novartis. Alessio Cortellini received consulting/advisory board fees from AstraZeneca, MSD, Roche, and BMS; and speakers’ fee from Novartis, Astellas, MSD, and AstraZeneca. Orazio Caffo received honoraria as a speaker by Astellas, Bayer, AstraZeneca, Janssen, Pfizer, and Sanofi, and a consultant for advisory role by Astellas, Janssen, and MSD. Paolo Andrea Zucali reports outside the submitted work personal fees for advisory role, speaker engagements, and travel and accommodation expenses from MSD, Astellas, Janssen, Sanofi, Ipsen, Pfizer, Novartis, BMS, Amgen, AstraZeneca, Roche, and Bayer. Camillo Porta received honoraria as a Consultant or Speaker for Angelini, AstraZeneca, BMS, Eisai, EUSA, General Electric, Ipsen, Janssen, Merck, MSD, Novartis, and Pfizer; acted as an Expert Testimony for EUSA and Pfizer; and was a Protocol Steering Committee Member of BMS, Eisai, and EUSA Pharma. Finally, he did receive travel support from Roche. All other authors have no conflict of interest to declare.
Figures

References
-
- World Health Organization. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update, 2020, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
-
- Covid-19, i casi in Italia: 21 giugno ore 18, https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioNotizieNuovo...
LinkOut - more resources
Full Text Sources
Miscellaneous

