Implantable Electrical Stimulation at Dorsal Root Ganglions Accelerates Osteoporotic Fracture Healing via Calcitonin Gene-Related Peptide
- PMID: 34708571
- PMCID: PMC8728818
- DOI: 10.1002/advs.202103005
Implantable Electrical Stimulation at Dorsal Root Ganglions Accelerates Osteoporotic Fracture Healing via Calcitonin Gene-Related Peptide
Abstract
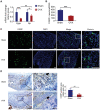
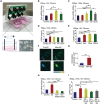
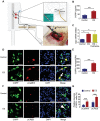
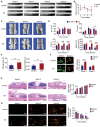
The neuronal engagement of the peripheral nerve system plays a crucial role in regulating fracture healing, but how to modulate the neuronal activity to enhance fracture healing remains unexploited. Here it is shown that electrical stimulation (ES) directly promotes the biosynthesis and release of calcitonin gene-related peptide (CGRP) by activating Ca2+ /CaMKII/CREB signaling pathway and action potential, respectively. To accelerate rat femoral osteoporotic fracture healing which presents with decline of CGRP, soft electrodes are engineered and they are implanted at L3 and L4 dorsal root ganglions (DRGs). ES delivered at DRGs for the first two weeks after fracture increases CGRP expression in both DRGs and fracture callus. It is also identified that CGRP is indispensable for type-H vessel formation, a biological event coupling angiogenesis and osteogenesis, contributing to ES-enhanced osteoporotic fracture healing. This proof-of-concept study shows for the first time that ES at lumbar DRGs can effectively promote femoral fracture healing, offering an innovative strategy using bioelectronic device to enhance bone regeneration.
Keywords: CGRP; bone regeneration; dorsal root ganglions; electrical stimulation.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dorsal root ganglion electrical stimulation promoted intertransverse process spinal fusion without decortications and bone grafting: a proof-of-concept study.Spine J. 2014 Oct 1;14(10):2472-8. doi: 10.1016/j.spinee.2014.04.001. Epub 2014 Apr 13. Spine J. 2014. PMID: 24735748
-
The influence of brain injury or peripheral nerve injury on calcitonin gene-related peptide concentration variation and fractures healing process.Artif Cells Blood Substit Immobil Biotechnol. 2009;37(2):85-91. doi: 10.1080/10731190902743149. Epub 2009 Feb 26. Artif Cells Blood Substit Immobil Biotechnol. 2009. PMID: 19247856
-
The Effects of Calcitonin Gene-Related Peptide on Bone Homeostasis and Regeneration.Curr Osteoporos Rep. 2020 Dec;18(6):621-632. doi: 10.1007/s11914-020-00624-0. Epub 2020 Oct 8. Curr Osteoporos Rep. 2020. PMID: 33030684 Review.
-
Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats.Nat Med. 2016 Oct;22(10):1160-1169. doi: 10.1038/nm.4162. Epub 2016 Aug 29. Nat Med. 2016. PMID: 27571347 Free PMC article.
-
[Control of bone remodeling by nervous system. Neural involvement in fracture healing and bone regeneration].Clin Calcium. 2010 Dec;20(12):1820-7. Clin Calcium. 2010. PMID: 21123934 Review. Japanese.
Cited by
-
Remodeling of the Intra-Conduit Inflammatory Microenvironment to Improve Peripheral Nerve Regeneration with a Neuromechanical Matching Protein-Based Conduit.Adv Sci (Weinh). 2024 May;11(17):e2302988. doi: 10.1002/advs.202302988. Epub 2024 Mar 2. Adv Sci (Weinh). 2024. PMID: 38430538 Free PMC article.
-
Strategies for in situ tissue engineering of vascularized bone regeneration (Review).Biomed Rep. 2023 May 22;18(6):42. doi: 10.3892/br.2023.1625. eCollection 2023 Jun. Biomed Rep. 2023. PMID: 37325184 Free PMC article. Review.
-
Exploring the memory: existing activity-dependent tools to tag and manipulate engram cells.Front Cell Neurosci. 2024 Jan 8;17:1279032. doi: 10.3389/fncel.2023.1279032. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38259503 Free PMC article. Review.
-
Bioprinting of inorganic-biomaterial/neural-stem-cell constructs for multiple tissue regeneration and functional recovery.Natl Sci Rev. 2024 Jan 25;11(4):nwae035. doi: 10.1093/nsr/nwae035. eCollection 2024 Apr. Natl Sci Rev. 2024. PMID: 38463933 Free PMC article.
-
Editorial: New advances in functional rehabilitation after central and peripheral nervous system injury.Front Neurol. 2023 Mar 16;14:1160382. doi: 10.3389/fneur.2023.1160382. eCollection 2023. Front Neurol. 2023. PMID: 37006476 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
