Patients' attitude towards a sham-controlled trial on pulmonary vein isolation in atrial fibrillation
- PMID: 34709451
- PMCID: PMC8766391
- DOI: 10.1007/s00392-021-01959-z
Patients' attitude towards a sham-controlled trial on pulmonary vein isolation in atrial fibrillation
Abstract
Background: The interpretation of recent trials on pulmonary vein ablation (PVI) for the treatment of atrial fibrillation (AF) is hampered by the lack of blinding and sham controls. The feasibility of a sham-controlled trial has been questioned. We aimed to assess the attitude of potential participants regarding a sham-controlled trial in a common AF-patient population planned for PVI.
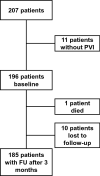
Methods: Patients in two tertiary care centres planned for PVI were asked for their current AF symptoms using the Atrial Fibrillation Effect on QualiTy of Life (AFEQT) questionnaire 1 day before catheter ablation. Subsequently, the study design of a hypothetical sham-controlled PVI-study was introduced, and patients were asked for their agreement in participation. Telephone follow-up of the AFEQT questionnaire was conducted 3 months after PVI.
Results: One hundred and ninety-six patients (mean age 64 ± 11 years, 63% male) were included. Seventy-nine (40%) patients expressed their agreement to participate in the hypothetical sham-controlled trial. An additional 7% agreed to participate if a cross-over option after three months was offered. Agreement rate was similar in patients with first and Redo-PVI and minimal, moderate or severe symptoms. Mean overall AFEQT at baseline was 55 ± 19 and improved by 25 ± 20 points after 3 months (p < 0.001 versus baseline).
Conclusion: With a participation rate of 40% in potential study participants, a sham-controlled trial for pulmonary vein isolation seems feasible. Patient-reported symptom relief after pulmonary vein isolation is in accordance with previous randomized open studies. The benefit of PVI should be rigorously evaluated in a sham-controlled trial.
Keywords: Atrial fibrillation; Catheter ablation; Pulmonary vein isolation; Sham-controlled trial.
© 2021. The Author(s).
Conflict of interest statement
None.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

