Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center
- PMID: 34709989
- PMCID: PMC8555518
- DOI: 10.1080/19490976.2021.1994834
Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center
Abstract
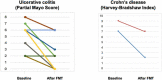
Inflammatory bowel disease (IBD) is a risk factor for C. difficile infection (CDI), which, in turn, complicates the clinical course of IBD. Fecal microbiota transplantation (FMT) is safe and effective in patients with IBD and recurrent CDI (rCDI). In our study, patients with IBD and rCDI received FMT by colonoscopy and were followed-up for 8 weeks. The primary outcome was negative C. difficile toxin 8 weeks after FMT. Eighteen patients with IBD were enrolled. Eight patients received sequential FMT either for pseudomembranous colitis or failure of single fecal infusion. At 8-week follow-up the C. difficile toxin was negative in 17 patients, and most (83%) experienced also improvement of IBD disease activity. Overall, we did not observe any serious adverse event.FMT appears to be highly effective and safe in patients with IBD and rCDI and is likely not only to eradicate CDI but also to improve disease activity of IBD.
Keywords: Clostridioides difficile infection; Crohn’s disease; Fecal microbiota transplantation; gut microbiota; inflammatory bowel disease; microbiome; ulcerative colitis.
Conflict of interest statement
AG reports personal fees for consultancy for Eisai S.r.l., 3PSolutions, Real Time Meeting, Fondazione Istituto Danone, Sinergie S.r.l. Board MRGE, and SanofiS.p.A, personal fees for acting as a speaker for Takeda S.p.A, AbbVie, and Sandoz S.p.A, and personal fees for acting on advisory boards for VSL3 and Eisai. FS has received personal fees for acting as advisor for
Abbvie, Janssen, MSD, Sanofi and Takeda. GC has received personal fees for acting as advisor for Ferring Therapeutics. GI has received personal fees for acting as speaker for Biocodex, Danone, Metagenics, and for acting as consultant/advisor for Ferring Therapeutics, Giuliani, Metagenics. None of other authors has any competing interest to disclose.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources