Attenuating persistent sodium current-induced atrial myopathy and fibrillation by preventing mitochondrial oxidative stress
- PMID: 34710060
- PMCID: PMC8675199
- DOI: 10.1172/jci.insight.147371
Attenuating persistent sodium current-induced atrial myopathy and fibrillation by preventing mitochondrial oxidative stress
Abstract
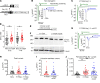
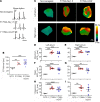
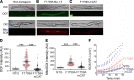
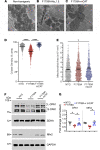
Mechanistically driven therapies for atrial fibrillation (AF), the most common cardiac arrhythmia, are urgently needed, the development of which requires improved understanding of the cellular signaling pathways that facilitate the structural and electrophysiological remodeling that occurs in the atria. Similar to humans, increased persistent Na+ current leads to the development of an atrial myopathy and spontaneous and long-lasting episodes of AF in mice. How increased persistent Na+ current causes both structural and electrophysiological remodeling in the atria is unknown. We crossbred mice expressing human F1759A-NaV1.5 channels with mice expressing human mitochondrial catalase (mCAT). Increased expression of mCAT attenuated mitochondrial and cellular reactive oxygen species (ROS) and the structural remodeling that was induced by persistent F1759A-Na+ current. Despite the heterogeneously prolonged atrial action potential, which was unaffected by the reduction in ROS, the incidences of spontaneous AF, pacing-induced after-depolarizations, and AF were substantially reduced. Expression of mCAT markedly reduced persistent Na+ current-induced ryanodine receptor oxidation and dysfunction. In summary, increased persistent Na+ current in atrial cardiomyocytes, which is observed in patients with AF, induced atrial enlargement, fibrosis, mitochondrial dysmorphology, early after-depolarizations, and AF, all of which can be attenuated by resolving mitochondrial oxidative stress.
Keywords: Arrhythmias; Cardiology; Cardiovascular disease; Sodium channels.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

