Severity of Disease Among Adults Hospitalized with Laboratory-Confirmed COVID-19 Before and During the Period of SARS-CoV-2 B.1.617.2 (Delta) Predominance - COVID-NET, 14 States, January-August 2021
- PMID: 34710076
- PMCID: PMC8553023
- DOI: 10.15585/mmwr.mm7043e1
Severity of Disease Among Adults Hospitalized with Laboratory-Confirmed COVID-19 Before and During the Period of SARS-CoV-2 B.1.617.2 (Delta) Predominance - COVID-NET, 14 States, January-August 2021
Abstract
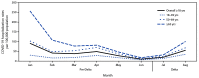
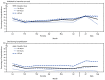
In mid-June 2021, B.1.671.2 (Delta) became the predominant variant of SARS-CoV-2, the virus that causes COVID-19, circulating in the United States. As of July 2021, the Delta variant was responsible for nearly all new SARS-CoV-2 infections in the United States.* The Delta variant is more transmissible than previously circulating SARS-CoV-2 variants (1); however, whether it causes more severe disease in adults has been uncertain. Data from the CDC COVID-19-Associated Hospitalization Surveillance Network (COVID-NET), a population-based surveillance system for COVID-19-associated hospitalizations, were used to examine trends in severe outcomes in adults aged ≥18 years hospitalized with laboratory-confirmed COVID-19 during periods before (January-June 2021) and during (July-August 2021) Delta variant predominance. COVID-19-associated hospitalization rates among all adults declined during January-June 2021 (pre-Delta period), before increasing during July-August 2021 (Delta period). Among sampled nonpregnant hospitalized COVID-19 patients with completed medical record abstraction and a discharge disposition during the pre-Delta period, the proportion of patients who were admitted to an intensive care unit (ICU), received invasive mechanical ventilation (IMV), or died while hospitalized did not significantly change from the pre-Delta period to the Delta period. The proportion of hospitalized COVID-19 patients who were aged 18-49 years significantly increased, from 24.7% (95% confidence interval [CI] = 23.2%-26.3%) of all hospitalizations in the pre-Delta period, to 35.8% (95% CI = 32.1%-39.5%, p<0.01) during the Delta period. When examined by vaccination status, 71.8% of COVID-19-associated hospitalizations in the Delta period were in unvaccinated adults. Adults aged 18-49 years accounted for 43.6% (95% CI = 39.1%-48.2%) of all hospitalizations among unvaccinated adults during the Delta period. No difference was observed in ICU admission, receipt of IMV, or in-hospital death among nonpregnant hospitalized adults between the pre-Delta and Delta periods. However, the proportion of unvaccinated adults aged 18-49 years hospitalized with COVID-19 has increased as the Delta variant has become more predominant. Lower vaccination coverage in this age group likely contributed to the increase in hospitalized patients during the Delta period. COVID-19 vaccination is critical for all eligible adults, including those aged <50 years who have relatively low vaccination rates compared with older adults.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Evan J. Anderson reports grants from Pfizer, Merck, PaxVax, Micron, Sanofi-Pasteur, Janssen, MedImmune, and GlaxoSmithKline; personal fees from Pfizer, Medscape, Kentucky Bioprocessing, Inc., Sanofi-Pasteur, and Janssen, outside the submitted work; and institutional funding from the National Institutes of Health to conduct clinical trials of Moderna and Janssen COVID-19 vaccines. Laurie M. Billing and Eli Shiltz report grants from the Council of State and Territorial Epidemiologists during the conduct of the study. Ruth Lynfield reports editorial fees from the American Academy of Pediatrics Red Book (Committee on Infectious Diseases), which were donated to the Minnesota Department of Health. William Schaffner reports personal fees from VBI Vaccines, outside the submitted work. No other potential conflicts of interest were disclosed.
Figures
References
-
- Delahoy MJ, Whitaker M, O’Halloran A, et al.; COVID-NET Surveillance Team. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19—COVID-NET, 13 states, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1347–54. 10.15585/mmwr.mm6938e1 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

