Vil-Cre specific Schlafen 3 knockout mice exhibit sex-specific differences in intestinal differentiation markers and Schlafen family members expression levels
- PMID: 34710177
- PMCID: PMC8553116
- DOI: 10.1371/journal.pone.0259195
Vil-Cre specific Schlafen 3 knockout mice exhibit sex-specific differences in intestinal differentiation markers and Schlafen family members expression levels
Abstract
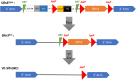
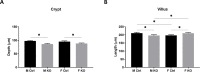
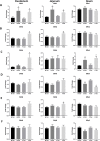
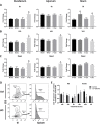
The intestinal epithelium requires self-renewal and differentiation in order to function and adapt to pathological diseases such as inflammatory bowel disease, short gut syndrome, and ulcers. The rodent Slfn3 protein and the human Slfn12 analog are known to regulate intestinal epithelial differentiation. Previous work utilizing a pan-Slfn3 knockout (KO) mouse model revealed sex-dependent gene expression disturbances in intestinal differentiation markers, metabolic pathways, Slfn family member mRNA expression, adaptive immune cell proliferation/functioning genes, and phenotypically less weight gain and sex-dependent changes in villus length and crypt depth. We have now created a Vil-Cre specific Slfn3KO (VC-Slfn3KO) mouse to further evaluate its role in intestinal differentiation. There were increases in Slfn1, Slfn2, Slfn4, and Slfn8 and decreases in Slfn5 and Slfn9 mRNA expression that were intestinal region and sex-specific. Differentiation markers, sucrase isomaltase (SI), villin 1, and dipeptidyl peptidase 4 and glucose transporters, glucose transporter 1 (Glut1), Glut2, and sodium glucose transporter 1 (SGLT1), were increased in expression in VC-Slfn3KO mice based on intestinal region and were also highly female sex-biased, except for SI in the ileum was also increased for male VC-Slfn3KO mice and SGLT1 was decreased for both sexes. Overall, the variations that we observed in these VC-Slfn3KO mice indicate a complex regulation of intestinal gene expression that is sex-dependent.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bustos O, Naik S, Ayers G, Casola C, Perez-Lamigueiro MA, Chippindale PT, et al. Evolution of the Schlafen genes, a gene family associated with embryonic lethality, meiotic drive, immune processes and orthopoxvirus virulence. Gene. 2009;447(1):1–11. doi: 10.1016/j.gene.2009.07.006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

