Reducing surgical site infections in low-income and middle-income countries (FALCON): a pragmatic, multicentre, stratified, randomised controlled trial
- PMID: 34710362
- PMCID: PMC8586736
- DOI: 10.1016/S0140-6736(21)01548-8
Reducing surgical site infections in low-income and middle-income countries (FALCON): a pragmatic, multicentre, stratified, randomised controlled trial
Abstract
Background: Surgical site infection (SSI) is the most common postoperative complication worldwide. WHO guidelines to prevent SSI recommend alcoholic chlorhexidine skin preparation and fascial closure using triclosan-coated sutures, but called for assessment of both interventions in low-resource settings. This study aimed to test both interventions in low-income and middle-income countries.
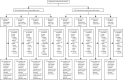
Methods: FALCON was a 2 × 2 factorial, randomised controlled trial stratified by whether surgery was clean-contaminated, or contaminated or dirty, including patients undergoing abdominal surgery with a skin incision of 5 cm or greater. This trial was undertaken in 54 hospitals in seven countries (Benin, Ghana, India, Mexico, Nigeria, Rwanda, and South Africa). Patients were computer randomised 1:1:1:1 to: (1) 2% alcoholic chlorhexidine and non-coated suture, (2) 2% alcoholic chlorhexidine and triclosan-coated suture, (3) 10% aqueous povidone-iodine and non-coated suture, or (4) 10% aqueous povidone-iodine and triclosan-coated suture. Patients and outcome assessors were masked to intervention allocation. The primary outcome was SSI, reported by trained outcome assessors, and presented using adjusted relative risks and 95% CIs. Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, NCT03700749.
Findings: Between Dec 10, 2018, and Sept 7, 2020, 5788 patients (3091 in clean-contaminated stratum, 2697 in contaminated or dirty stratum) were randomised (1446 to alcoholic chlorhexidine and non-coated suture, 1446 to alcoholic chlorhexidine and triclosan-coated suture, 1447 to aqueous povidone-iodine and non-coated suture, and 1449 to aqueous povidone-iodine and triclosan-coated suture). 14·0% (810/5788) of patients were children and 66·9% (3873/5788) had emergency surgery. The overall SSI rate was 22·0% (1163/5284; clean-contaminated stratum 15·5% [454/2923], contaminated or dirty stratum 30·0% [709/2361]). For both strata, there was no evidence of a difference in the risk of SSI with alcoholic chlorhexidine versus povidone-iodine (clean-contaminated stratum 15·3% [223/1455] vs 15·7% [231/1468], relative risk 0·97 [95% CI 0·82-1·14]; contaminated or dirty stratum 28·3% [338/1194] vs 31·8% [371/1167], relative risk 0·91 [95% CI 0·81-1·02]), or with triclosan-coated sutures versus non-coated sutures (clean-contaminated stratum 14·7% [215/1459] vs 16·3% [239/1464], relative risk 0·90 [95% CI 0·77-1·06]; contaminated or dirty stratum 29·4% [347/1181] vs 30·7% [362/1180], relative risk 0·98 [95% CI 0·87-1·10]). With both strata combined, there were no differences using alcoholic chlorhexidine or triclosan-coated sutures.
Interpretation: This trial did not show benefit from 2% alcoholic chlorhexidine skin preparation compared with povidone-iodine, or with triclosan-coated sutures compared with non-coated sutures, in preventing SSI in clean-contaminated or contaminated or dirty surgical wounds. Both interventions are more expensive than alternatives, and these findings do not support recommendations for routine use.
Funding: National Institute for Health Research (NIHR) Global Health Research Unit Grant, BD.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

Comment in
-
Prevention of surgical site infection in low-resource settings.Lancet. 2021 Nov 6;398(10312):1664-1665. doi: 10.1016/S0140-6736(21)01695-0. Epub 2021 Oct 25. Lancet. 2021. PMID: 34710361 No abstract available.
-
Optimal usage of antibacterial sutures for wound closure in clinical trials addressing SSI.Lancet. 2023 May 6;401(10387):1497-1498. doi: 10.1016/S0140-6736(23)00668-2. Lancet. 2023. PMID: 37149304 No abstract available.
References
-
- Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. - PubMed
-
- WHO Report on the burden of endemic health care-associated infection worldwide, a systematic review of the literature. 2011. https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng...
-
- European Centre for Disease Prevention and Control Annual Epidemiological Report for 2017. 2019. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-SS...
-
- Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96:1–15. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

