Ex Vivo Plasmodium malariae Culture Method for Antimalarial Drugs Screen in the Field
- PMID: 34711047
- PMCID: PMC9974065
- DOI: 10.1021/acsinfecdis.1c00262
Ex Vivo Plasmodium malariae Culture Method for Antimalarial Drugs Screen in the Field
Abstract
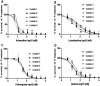
In vitro and ex vivo cultivation of Plasmodium (P) falciparum has facilitated active research into the malaria parasite toward the quest for basic knowledge and the discovery of effective drug treatments. Such a drug discovery program is currently difficult for P. malariae simply because of the absence of in vitro and ex vivo cultivation system for its asexual blood stages supporting antimalarial evaluation. Despite availability of artemisinin combination therapies effective on P. falciparum, P. malariae is being increasingly detected in malaria endemic countries. P. malariae is responsible for chronic infections and is associated with a high burden of anemia and morbidity. Here, we optimized and adapted ex vivo conditions under which P. malariae can be cultured and used for screening antimalarial drugs. Subsequently, this enabled us to test compounds such as artemether, chloroquine, lumefantrine, and quinine for ex vivo antimalarial activity against P. malariae.
Keywords: Plasmodium malariae; drug discovery; ex vivo culture; malaria; nonfalciparum.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Calderaro A.; Piccolo G.; Gorrini C.; Rossi S.; Montecchini S.; Dell’Anna M. L.; De Conto F.; Medici M. C.; Chezzi C.; Arcangeletti M. C. Accurate Identification of the Six Human Plasmodium Spp. Causing Imported Malaria, Including Plasmodium Ovale Wallikeri and Plasmodium Knowlesi. Malar. J. 2013, 12, 321.10.1186/1475-2875-12-321. - DOI - PMC - PubMed
-
- Imwong M.; Madmanee W.; Suwannasin K.; Kunasol C.; Peto T. J.; Tripura R.; Von Seidlein L.; Nguon C.; Davoeung C.; Day N. P. J.; Dondorp A. M.; White N. J. Asymptomatic Natural Human Infections with the Simian Malaria Parasites Plasmodium Cynomolgi and Plasmodium Knowlesi. J. Infect. Dis. 2019, 219 (5), 695–702. 10.1093/infdis/jiy519. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

