Volumetric 23Na Single and Triple-Quantum Imaging at 7T: 3D-CRISTINA
- PMID: 34711477
- PMCID: PMC9948835
- DOI: 10.1016/j.zemedi.2021.09.001
Volumetric 23Na Single and Triple-Quantum Imaging at 7T: 3D-CRISTINA
Erratum in
-
Erratum to "Volumetric 23Na single and triple-quantum imaging at 7T: 3D-CRISTINA" [Z Med Phys 32 (2022) 199-208].Z Med Phys. 2025 Feb;35(1):113. doi: 10.1016/j.zemedi.2024.07.008. Epub 2024 Sep 2. Z Med Phys. 2025. PMID: 39227227 Free PMC article. No abstract available.
Abstract
Purpose: To measure multi-quantum coherence (MQC) 23Na signals for noninvasive cell physiological information in the whole-brain, the 2D-CRISTINA method was extended to 3D. This experimental study investigated the use and results of a new sequence, 3D-CRISTINA, on a phantom and healthy volunteers.
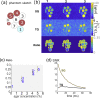
Methods: The 3D Cartesian single and triple-quantum imaging of 23Na (3D-CRISTINA) was developed and implemented at 7T, favoring a non-selective volume excitation for increased signal-to-noise ratio (SNR) and lower energy deployment than its 2D counterpart. Two independent phase cycles were used in analogy to the 2D method. A comparison of 6-steps cycles and 12-steps cycles was performed. We used a phantom composed of different sodium and agarose concentrations, 50mM to 150mM Na+, and 0-5% agarose for sequence validation. Four healthy volunteers were scanned at 7T for whole brain MQC imaging. The sequence 3D-CRISTINA was developed and tested at 7T.
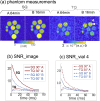
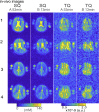
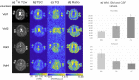
Results: At 7T, the 3D-CRISTINA acquisition allowed to reduce the TR to 230ms from the previous 390ms for 2D, resulting in a total acquisition time of 53min for a 3D volume of 4×4×8mm resolution. The phase cycle evaluation showed that the 7T acquisition time could be reduced by 4-fold with moderate single and triple-quantum signals SNR loss. The healthy volunteers demonstrated clinical feasibility at 7T and showed a difference in the MQC signals ratio of White Matter (WM) and Grey Matter (GM).
Conclusion: Volumetric CRISTINA multi-quantum imaging allowed whole-brain coverage. The non-selective excitation enabled a faster scan due to a decrease in energy deposition which enabled a lower repetition time. Thus, it should be the preferred choice for future in vivo multi-quantum applications compared to the 2D method. A more extensive study is warranted to explore WM and GM MQC differences.
Keywords: Sodium MRI; Sodium triple-quantum imaging; Whole-brain imaging.
Copyright © 2021. Published by Elsevier GmbH.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

