Heterogeneity of Circulating Tumor Cell-Associated Genomic Gains in Breast Cancer and Its Association with the Host Immune Response
- PMID: 34711609
- PMCID: PMC9397625
- DOI: 10.1158/0008-5472.CAN-21-1079
Heterogeneity of Circulating Tumor Cell-Associated Genomic Gains in Breast Cancer and Its Association with the Host Immune Response
Abstract
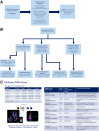
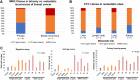
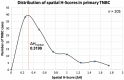
Tumor cells that preferentially enter circulation include the precursors of metastatic cancer. Previously, we characterized circulating tumor cells (CTC) from patients with breast cancer and identified a signature of genomic regions with recurrent copy-number gains. Through FISH, we now show that these CTC-associated regions are detected within the matched untreated primary tumors of these patients (21% to 69%, median 55.5%, n = 19). Furthermore, they are more prevalent in the metastases of patients who died from breast cancer after multiple rounds of treatment (70% to 100%, median 93%, samples n = 41). Diversity indices revealed that higher spatial heterogeneity for these regions within primary tumors is associated with increased dissemination and metastasis. An identified subclone with multiple regions gained (MRG clone) was enriched in a posttreatment primary breast carcinoma as well as multiple metastatic tumors and local breast recurrences obtained at autopsy, indicative of a distinct early subclone with the capability to resist multiple lines of treatment and eventually cause death. In addition, multiplex immunofluorescence revealed that tumor heterogeneity is significantly associated with the degree of infiltration of B lymphocytes in triple-negative breast cancer, a subtype with a large immune component. Collectively, these data reveal the functional potential of genetic subclones that comprise heterogeneous primary breast carcinomas and are selected for in CTCs and posttreatment breast cancer metastases. In addition, they uncover a relationship between tumor heterogeneity and host immune response in the tumor microenvironment. SIGNIFICANCE: As breast cancers progress, they become more heterogeneous for multiple regions amplified in circulating tumor cells, and intratumoral spatial heterogeneity is associated with the immune landscape.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol 2015;12:381–94. - PubMed
-
- O'Connell P, Pekkel V, Allred DC, Fuqua SAW, Osborne CK, Clark GM. Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst 1998;90:697–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

