Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells
- PMID: 34711700
- PMCID: PMC9148840
- DOI: 10.15283/ijsc21067
Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells
Abstract
Background and objectives: Despite advances in wound treatments, chronic diabetic wounds remain a significant medical challenge. Exosomes from mesenchymal stem cells (MSCs) and small molecule activators of nuclear factor erythroid 2-related factor 2 (Nrf2) have emerged as potential therapies for nonhealing diabetic wounds. This study aimed to evaluate the effects of exosomes from bone marrow-derived MSCs (BMSCs) alone, or in combination with a small molecule activator of Nrf2 on diabetic wound healing.
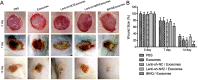
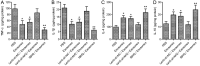
Methods and results: BMSCs and endothelial progenitor cells (EPCs) were isolated from the femur and tibia bone marrow of Sprague-Dawley (SD) rats and culture-expanded. Exosomes were harvested from the BMSC culture supernatants through ultracentrifugation. The effects of the exosomes and Nrf2 knockdown, alone or in combination, on EPC tube formation were evaluated. Streptozotocin-induced diabetic rats bearing a fresh full-thickness round wound were treated with the exosomes alone, or in combination with a lentiviral shRNA targeting Nrf2 (Lenti-sh-Nrf2) or tert-butylhydroquinone (tBHQ), a small molecule activator of Nrf2. Two weeks later, wound closure, re-epithelization, collagen deposition, neovascularization, and local inflammation were evaluated. BMSC exosomes promoted while Nrf2 knockdown inhibited EPC tube formation. BMSC exosomes accelerated wound closure, re-epithelization, collagen deposition, and neovascularization, and reduced wound inflammation in diabetic rats. These regenerative and anti-inflammatory effects of the exosomes were inhibited by Lenti-sh-Nrf2 but enhanced by tBHQ administration.
Conclusions: BMSC exosomes in combination with a small molecule Nrf2 activator hold promise as a new therapeutic option for chronic diabetic wounds.
Keywords: Bone marrow-derived mesenchymal stem cells; Diabetic wound healing; Exosomes; Nrf2; Tert-Butylhydroquinone.
Conflict of interest statement
The authors have no conflicting financial interest.
Figures




Similar articles
-
The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review.Curr Issues Mol Biol. 2022 Oct 16;44(10):4960-4976. doi: 10.3390/cimb44100337. Curr Issues Mol Biol. 2022. PMID: 36286052 Free PMC article. Review.
-
Mesenchymal stem cell-derived exosomes: The dawn of diabetic wound healing.World J Diabetes. 2022 Dec 15;13(12):1066-1095. doi: 10.4239/wjd.v13.i12.1066. World J Diabetes. 2022. PMID: 36578867 Free PMC article. Review.
-
Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis.J Nanobiotechnology. 2021 May 21;19(1):150. doi: 10.1186/s12951-021-00894-5. J Nanobiotechnology. 2021. PMID: 34020670 Free PMC article.
-
Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway.Stem Cell Res Ther. 2020 Aug 12;11(1):350. doi: 10.1186/s13287-020-01824-2. Stem Cell Res Ther. 2020. PMID: 32787917 Free PMC article.
-
Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated miR-21-5p.Int J Nanomedicine. 2020 Oct 19;15:7979-7993. doi: 10.2147/IJN.S275650. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116513 Free PMC article.
Cited by
-
The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review.Curr Issues Mol Biol. 2022 Oct 16;44(10):4960-4976. doi: 10.3390/cimb44100337. Curr Issues Mol Biol. 2022. PMID: 36286052 Free PMC article. Review.
-
Mesenchymal stem cell-derived exosomes: The dawn of diabetic wound healing.World J Diabetes. 2022 Dec 15;13(12):1066-1095. doi: 10.4239/wjd.v13.i12.1066. World J Diabetes. 2022. PMID: 36578867 Free PMC article. Review.
-
Small extracellular vesicles from mesenchymal stem cells: A potential Weapon for chronic non-healing wound treatment.Front Bioeng Biotechnol. 2023 Jan 10;10:1083459. doi: 10.3389/fbioe.2022.1083459. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36704302 Free PMC article. Review.
-
Nrf2 activation: a key mechanism in stem cell exosomes-mediated therapies.Cell Mol Biol Lett. 2024 Mar 2;29(1):30. doi: 10.1186/s11658-024-00551-3. Cell Mol Biol Lett. 2024. PMID: 38431569 Free PMC article. Review.
-
Regulation of the Nrf2/HO-1 axis by mesenchymal stem cells-derived extracellular vesicles: implications for disease treatment.Front Cell Dev Biol. 2024 Jun 10;12:1397954. doi: 10.3389/fcell.2024.1397954. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38915448 Free PMC article. Review.
References
-
- Hoang DH, Nguyen TD, Nguyen HP, Nguyen XH, Do PTX, Dang VD, Dam PTM, Bui HTH, Trinh MQ, Vu DM, Hoang NTM, Thanh LN, Than UTT. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci. 2020;7:119. doi: 10.3389/fmolb.2020.00119.d8551c1647974a088970fa3aafd4b75b - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

