A SARS-CoV-2 spike ferritin nanoparticle vaccine protects hamsters against Alpha and Beta virus variant challenge
- PMID: 34711815
- PMCID: PMC8553838
- DOI: 10.1038/s41541-021-00392-7
A SARS-CoV-2 spike ferritin nanoparticle vaccine protects hamsters against Alpha and Beta virus variant challenge
Abstract
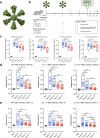
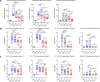
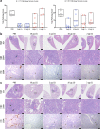
The emergence of SARS-CoV-2 variants of concern (VOC) requires adequate coverage of vaccine protection. We evaluated whether a SARS-CoV-2 spike ferritin nanoparticle vaccine (SpFN), adjuvanted with the Army Liposomal Formulation QS21 (ALFQ), conferred protection against the Alpha (B.1.1.7), and Beta (B.1.351) VOCs in Syrian golden hamsters. SpFN-ALFQ was administered as either single or double-vaccination (0 and 4 week) regimens, using a high (10 μg) or low (0.2 μg) dose. Animals were intranasally challenged at week 11. Binding antibody responses were comparable between high- and low-dose groups. Neutralizing antibody titers were equivalent against WA1, B.1.1.7, and B.1.351 variants following two high dose vaccinations. Dose-dependent SpFN-ALFQ vaccination protected against SARS-CoV-2-induced disease and viral replication following intranasal B.1.1.7 or B.1.351 challenge, as evidenced by reduced weight loss, lung pathology, and lung and nasal turbinate viral burden. These data support the development of SpFN-ALFQ as a broadly protective, next-generation SARS-CoV-2 vaccine.
© 2021. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Drs. Modjarrad and Joyce are co-inventors on a pending patent application for the Spike Protein Ferritin Nanoparticle (PCT/US2021/021405) Assigned to the U.S. Government as represented by the Secretary of the Army.
Figures

Update of
-
A SARS-CoV-2 spike ferritin nanoparticle vaccine protects against heterologous challenge with B.1.1.7 and B.1.351 virus variants in Syrian golden hamsters.bioRxiv [Preprint]. 2021 Jun 16:2021.06.16.448525. doi: 10.1101/2021.06.16.448525. bioRxiv. 2021. Update in: NPJ Vaccines. 2021 Oct 28;6(1):129. doi: 10.1038/s41541-021-00392-7. PMID: 34159328 Free PMC article. Updated. Preprint.
References
-
- Craven, J. (Regulatory Affairs Professionals Society, 2021). https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vac....
-
- CDC. SARS-CoV-2 Variant Classifications and Definitions, https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveill... (2021).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

