The impact of the timely birth dose vaccine on the global elimination of hepatitis B
- PMID: 34711822
- PMCID: PMC8553835
- DOI: 10.1038/s41467-021-26475-6
The impact of the timely birth dose vaccine on the global elimination of hepatitis B
Abstract
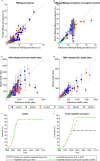
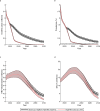
In 2016 the World Health Organization set the goal of eliminating hepatitis B globally by 2030. Horizontal transmission has been greatly reduced in most countries by scaling up coverage of the infant HBV vaccine series, and vertical transmission is therefore becoming increasingly dominant. Here we show that scaling up timely hepatitis B birth dose vaccination to 90% of new-borns in 110 low- and middle-income countries by 2030 could prevent 710,000 (580,000 to 890,000) deaths in the 2020 to 2030 birth cohorts compared to status quo, with the greatest benefits in Africa. Maintaining this could lead to elimination by 2030 in the Americas, but not before 2059 in Africa. Drops in coverage due to disruptions in 2020 may lead to 15,000 additional deaths, mostly in South-East Asia and the Western Pacific. Delays in planned scale-up could lead to an additional 580,000 deaths globally in the 2020 to 2030 birth cohorts.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: This work was carried out as part of the Vaccine Impact Modelling Consortium (
Figures



References
-
- Global hepatitis report 2017 (World Health Organization, Geneva, 2017).
-
- Combating hepatitis B and C to reach elimination by 2030: advocacy brief (World Health Organization, Geneva, 2016).
-
- Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis (World Health Organization, Geneva, 2016).
-
- Hepatitis B vaccines: WHO position paper. in Weekly epidemiological record, Vol. 84 405–420 (World Health Organization, 2009).
-
- Hepatitis B vaccines: WHO position paper--recommendations. Vaccine28, 589–590 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

