Age-related immune alterations and cerebrovascular inflammation
- PMID: 34711943
- PMCID: PMC9046462
- DOI: 10.1038/s41380-021-01361-1
Age-related immune alterations and cerebrovascular inflammation
Abstract
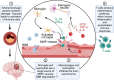
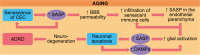
Aging is associated with chronic systemic inflammation, which contributes to the development of many age-related diseases, including vascular disease. The world's population is aging, leading to an increasing prevalence of both stroke and vascular dementia. The inflammatory response to ischemic stroke is critical to both stroke pathophysiology and recovery. Age is a predictor of poor outcomes after stroke. The immune response to stroke is altered in aged individuals, which contributes to the disparate outcomes between young and aged patients. In this review, we describe the current knowledge of the effects of aging on the immune system and the cerebral vasculature and how these changes alter the immune response to stroke and vascular dementia in animal and human studies. Potential implications of these age-related immune alterations on chronic inflammation in vascular disease outcome are highlighted.
© 2021. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

