Clinical impact and quality of randomized controlled trials involving interventions evaluating artificial intelligence prediction tools: a systematic review
- PMID: 34711955
- PMCID: PMC8553754
- DOI: 10.1038/s41746-021-00524-2
Clinical impact and quality of randomized controlled trials involving interventions evaluating artificial intelligence prediction tools: a systematic review
Abstract
The evidence of the impact of traditional statistical (TS) and artificial intelligence (AI) tool interventions in clinical practice was limited. This study aimed to investigate the clinical impact and quality of randomized controlled trials (RCTs) involving interventions evaluating TS, machine learning (ML), and deep learning (DL) prediction tools. A systematic review on PubMed was conducted to identify RCTs involving TS/ML/DL tool interventions in the past decade. A total of 65 RCTs from 26,082 records were included. A majority of them had model development studies and generally good performance was achieved. The function of TS and ML tools in the RCTs mainly included assistive treatment decisions, assistive diagnosis, and risk stratification, but DL trials were only conducted for assistive diagnosis. Nearly two-fifths of the trial interventions showed no clinical benefit compared to standard care. Though DL and ML interventions achieved higher rates of positive results than TS in the RCTs, in trials with low risk of bias (17/65) the advantage of DL to TS was reduced while the advantage of ML to TS disappeared. The current applications of DL were not yet fully spread performed in medicine. It is predictable that DL will integrate more complex clinical problems than ML and TS tools in the future. Therefore, rigorous studies are required before the clinical application of these tools.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
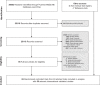
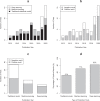

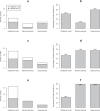
References
-
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. - PubMed
-
- Pencina MJ, Goldstein BA, D'Agostino RB. Prediction models-development, evaluation, and clinical application. N. Engl. J. Med. 2020;382:1583–1586. - PubMed
-
- Eagle KA, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. Jama. 2004;291:2727–2733. - PubMed
-
- Wilson PW, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

