Prophylactic Tocilizumab Prior to Anti-CD19 CAR-T Cell Therapy for Non-Hodgkin Lymphoma
- PMID: 34712233
- PMCID: PMC8546323
- DOI: 10.3389/fimmu.2021.745320
Prophylactic Tocilizumab Prior to Anti-CD19 CAR-T Cell Therapy for Non-Hodgkin Lymphoma
Abstract
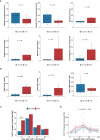
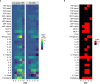
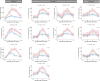
Anti-CD19 chimeric antigen receptor T (CAR-T) cells have demonstrated activity against relapsed/refractory lymphomas. Cytokine release syndrome (CRS) and immune effector cell - associated neurotoxicity syndrome (ICANS) are well-known complications. Tocilizumab, a monoclonal antibody targeting the interleukin-6 (IL-6) receptor was administered 1 hour prior to infusion of anti-CD19 CAR-T cells with CD3ζ/4-1BB costimulatory signaling used to treat non-Hodgkin lymphoma patients. Relapsed/refractory lymphoma patients treated with anti-CD19 CAR-T cells were included in this analysis. Cytokine plasma levels were measured by electrochemiluminescence before lymphodepleting chemotherapy, prior to infusion and then on days 2, 4,6, and 14 days after treatment. Twenty patients were treated. Cell products included locally manufactured anti-CD19 CAR-T (n=18) and tisagenlecleucel (n=2). There were no adverse events attributed to tocilizumab. Ten patients had grade 1-2 CRS at a median of 4 (range 3-7) days. There were no cases of grade ≥3 CRS. Five patients had ICANS, grade 1 (n=4) and grade 4 (n=1). Laboratory studies obtained prior to lymphodepleting chemotherapy were comparable between patients with and without CRS, except for interleukin (IL)-15 plasma concentrations. patients with CRS had higher post-infusion ferritin and C reactive protein, with more marked increases in inflammatory cytokines, including IL-6, IL-15, IFN-γ, fractalkine and MCP-1. Fifteen patients (75%) achieved CR and 2 (10%), PR. One-year OS and PFS estimates were 83% and 73%. Prophylactic tocilizumab was associated with low CRS incidence and severity. There were no adverse events associated with tocilizumab, no increase in frequency or severity of ICANS and excellent disease control and overall survival.
Keywords: CAR- T cells; cytokine release syndrome (CRS); lymphoma; prophylaxis; tocilizumab.
Copyright © 2021 Caimi, Pacheco Sanchez, Sharma, Otegbeye, Ahmed, Rojas, Patel, Kleinsorge Block, Schiavone, Zamborsky, Boughan, Hillian, Reese-Koc, Maschan, Dropulic, Sekaly and de Lima.
Conflict of interest statement
BD is a previous employee of Lentigen, a Miltenyi Biotec Company, and has Patents and Royalties related to CAR-T immunotherapy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Jacobson C, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. . Primary Analysis of Zuma-5: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients With Relapsed/Refractory (R/R) Indolent Non-Hodgkin Lymphoma (iNHL). Blood (2020) 136:40–1.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

