Animal Models of Ehlers-Danlos Syndromes: Phenotype, Pathogenesis, and Translational Potential
- PMID: 34712265
- PMCID: PMC8547655
- DOI: 10.3389/fgene.2021.726474
Animal Models of Ehlers-Danlos Syndromes: Phenotype, Pathogenesis, and Translational Potential
Abstract
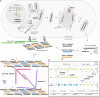
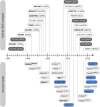
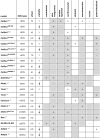
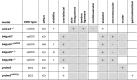
The Ehlers-Danlos syndromes (EDS) are a group of heritable connective tissues disorders mainly characterized by skin hyperextensibility, joint hypermobility and generalized tissue fragility. Currently, 14 EDS subtypes each with particular phenotypic features are recognized and are caused by genetic defects in 20 different genes. All of these genes are involved in the biosynthesis and/or fibrillogenesis of collagens at some level. Although great progress has been made in elucidating the molecular basis of different EDS subtypes, the pathogenic mechanisms underlying the observed phenotypes remain poorly understood, and consequentially, adequate treatment and management options for these conditions remain scarce. To date, several animal models, mainly mice and zebrafish, have been described with defects in 14 of the 20 hitherto known EDS-associated genes. These models have been instrumental in discerning the functions and roles of the corresponding proteins during development, maturation and repair and in portraying their roles during collagen biosynthesis and/or fibrillogenesis, for some even before their contribution to an EDS phenotype was elucidated. Additionally, extensive phenotypical characterization of these models has shown that they largely phenocopy their human counterparts, with recapitulation of several clinical hallmarks of the corresponding EDS subtype, including dermatological, cardiovascular, musculoskeletal and ocular features, as well as biomechanical and ultrastructural similarities in tissues. In this narrative review, we provide a comprehensive overview of animal models manifesting phenotypes that mimic EDS with a focus on engineered mouse and zebrafish models, and their relevance in past and future EDS research. Additionally, we briefly discuss domestic animals with naturally occurring EDS phenotypes. Collectively, these animal models have only started to reveal glimpses into the pathophysiological aspects associated with EDS and will undoubtably continue to play critical roles in EDS research due to their tremendous potential for pinpointing (common) signaling pathways, unveiling possible therapeutic targets and providing opportunities for preclinical therapeutic interventions.
Keywords: EDS; Ehlers–Danlos syndromes; animal models; mouse; zebrafish.
Copyright © 2021 Vroman, Malfait, Miller, Malfait and Syx.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Pain in the Ehlers-Danlos syndromes: Mechanisms, models, and challenges.Am J Med Genet C Semin Med Genet. 2021 Dec;187(4):429-445. doi: 10.1002/ajmg.c.31950. Epub 2021 Nov 19. Am J Med Genet C Semin Med Genet. 2021. PMID: 34797601 Review.
-
Pathogenic mechanisms in genetically defined Ehlers-Danlos syndromes.Trends Mol Med. 2024 Sep;30(9):824-843. doi: 10.1016/j.molmed.2024.06.001. Epub 2024 Aug 14. Trends Mol Med. 2024. PMID: 39147618 Review.
-
Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes.Dev Dyn. 2021 Mar;250(3):318-344. doi: 10.1002/dvdy.220. Epub 2020 Aug 17. Dev Dyn. 2021. PMID: 32629534 Free PMC article. Review.
-
The Ehlers-Danlos Syndromes against the Backdrop of Inborn Errors of Metabolism.Genes (Basel). 2022 Jan 29;13(2):265. doi: 10.3390/genes13020265. Genes (Basel). 2022. PMID: 35205310 Free PMC article. Review.
-
Vascular aspects of the Ehlers-Danlos Syndromes.Matrix Biol. 2018 Oct;71-72:380-395. doi: 10.1016/j.matbio.2018.04.013. Epub 2018 Apr 27. Matrix Biol. 2018. PMID: 29709596 Review.
Cited by
-
Identification of an ADAMTS2 frameshift variant in a cat family with Ehlers-Danlos syndrome.G3 (Bethesda). 2023 Aug 30;13(9):jkad152. doi: 10.1093/g3journal/jkad152. G3 (Bethesda). 2023. PMID: 37462293 Free PMC article.
-
Bioengineered vascular grafts with a pathogenic TGFBR1 variant model aneurysm formation in vivo and reveal underlying collagen defects.Sci Transl Med. 2024 May 8;16(746):eadg6298. doi: 10.1126/scitranslmed.adg6298. Epub 2024 May 8. Sci Transl Med. 2024. PMID: 38718134 Free PMC article.
-
Robust statistical assessment of Oncogenotype to Organotropism translation in xenografted zebrafish.bioRxiv [Preprint]. 2025 Jun 1:2025.05.28.656734. doi: 10.1101/2025.05.28.656734. bioRxiv. 2025. PMID: 40501949 Free PMC article. Preprint.
-
Current Evidence and Future Perspectives in the Medical Management of Vascular Ehlers-Danlos Syndrome: Focus on Vascular Prevention.J Clin Med. 2024 Jul 21;13(14):4255. doi: 10.3390/jcm13144255. J Clin Med. 2024. PMID: 39064294 Free PMC article. Review.
-
Patient-derived extracellular matrix demonstrates role of COL3A1 in blood vessel mechanics.Acta Biomater. 2023 Aug;166:346-359. doi: 10.1016/j.actbio.2023.05.015. Epub 2023 May 13. Acta Biomater. 2023. PMID: 37187299 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

