Knockout of PKC θ gene attenuates oleic acid-induced acute lung injury via reduction of inflammation and oxidative stress
- PMID: 34712430
- PMCID: PMC8528254
- DOI: 10.22038/ijbms.2021.56908.12695
Knockout of PKC θ gene attenuates oleic acid-induced acute lung injury via reduction of inflammation and oxidative stress
Abstract
Objectives: Acute respiratory distress syndrome resulting from acute lung injury has become a momentous clinical concern because of high morbidity and mortality in discharged patients with pulmonary and nonpulmonary diseases. This study aimed to explore the effect of protein kinase C (PKC) θ gene knockout on acute lung injury.
Materials and methods: Wt and PKC θ gene knockout mice were intravenously injected with oleic acid to induce acute lung injury. Pulmonary capillary permeability was assessed via measuring lung wet/dry weight ratio and level of protein in bronchoalveolar lavage fluid (BALF). Histological changes were used to examine acute lung injury. Malondialdehyde (MDA) level, superoxide dismutase (SOD) activity in serum, together with inflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α), were determined. Furthermore, the expressions of heme oxygenase (HO)-1, nuclear factor kappa B (NF κB), and inhibitor of NF-κB alpha (IκB α) were detected in the lungs.
Results: PKC θ gene knockout decreased lung wet/dry weight ratio, reduced levels of MDA, IL-6, and TNF-α in serum together with level of protein in BALF. Furthermore, PKC θ gene knockout increased the activities of SOD. Knockout of PKC θ was also observed to increase expression of HO-1 and reduce levels of p-NF κB and p-IKB α in the lungs.
Conclusion: These results suggest that PKC θ gene knockout attenuates oleic acid-induced acute lung injury via improving oxidative stress and inflammation.
Keywords: Acute lung injury; Gene knockout; Inflammation; Oleic acid; Oxidative stress; Protein kinase C θ.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

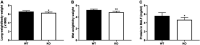



References
-
- Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–360. - PubMed
-
- Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047–1060. - PubMed
LinkOut - more resources
Full Text Sources
