PERSONAL: Feasibility Study Protocol for Placebo-Controlled, Randomized n-of-1 Trials of Tamsulosin for Lower Urinary Tract Symptoms
- PMID: 34713020
- PMCID: PMC8521798
- DOI: 10.3389/fdgth.2020.00007
PERSONAL: Feasibility Study Protocol for Placebo-Controlled, Randomized n-of-1 Trials of Tamsulosin for Lower Urinary Tract Symptoms
Abstract
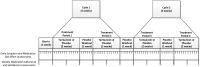
Background: Lower urinary tract symptoms (LUTS) affect more than half of men over age 70 and contribute to both poor health-related quality of life and polypharmacy. Tamsulosin hydrochloride, a selective α1-blocker, is the most common medication used to treat LUTS due to presumed benign prostatic hyperplasia and is often prescribed indefinitely, although not all men benefit from long-term therapy. N-of-1 trials allow for individualized estimates of benefit and harm and could facilitate decisions regarding chronic tamsulosin therapy for LUTS, particularly among older men. Our team developed the PERSONAL (PlacEbo-controlled, Randomized, patient-Selected Outcomes, N-of-1 triALs) app to track daily urinary symptoms and medication side effects for n-of-1 trials among older men with LUTS. Materials and Methods: We will conduct a feasibility study of 20 individual randomized n-of-1 trials using the PERSONAL app to compare tamsulosin (0.4 or 0.8 mg) vs. placebo among older men taking tamsulosin for LUTS. We will include men over age 65 with a smartphone for whom temporary discontinuation of tamsulosin is safe, (e.g., no history of acute retention). Participants will work with research staff to prospectively identify the most important urinary symptoms and medication side effects that they would like to digitally track. Men will then be randomized to 2-week treatment periods of tamsulosin or placebo followed by a 1-week wash-out with placebo, for 4 distinct treatment periods and 3 wash-out periods, totaling 11 weeks. Study medications will be blinded using over-encapsulation of tamsulosin pills and matching placebo. Our primary outcomes for this study will be recruitment and retention of eligible men, completion rates of n-of-1 trials and daily questionnaires using the PERSONAL app, and participants' perceived usefulness of their n-of-1 trial for determining whether tamsulosin is effective for them. Linear mixed effects models with individual-specific intercepts and intervention effects will also be used to estimate within-individual effects of tamsulosin. Discussion: The goal of this innovative study is to establish feasibility and acceptability of using a mobile health app and n-of-1 trials to provide older men with individualized estimates of benefits and harms of chronic tamsulosin therapy for LUTS.
Keywords: benign prostatic hyperplasia; deprescribing; medication side effects; patient-reported outcomes; personalized medicine; randomized clinical trial design; α-antagonist.
Copyright © 2020 Bauer, Breyer, Oni-Orisan, Steinman, Sim, McCulloch and Kenfield.
Figures


Similar articles
-
Tracking Lower Urinary Tract Symptoms and Tamsulosin Side Effects Among Older Men Using a Mobile App (PERSONAL): Feasibility and Usability Study.JMIR Form Res. 2021 Dec 10;5(12):e30762. doi: 10.2196/30762. JMIR Form Res. 2021. PMID: 34889745 Free PMC article.
-
Treatment satisfaction with tadalafil or tamsulosin vs placebo in men with lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH): results from a randomised, placebo-controlled study.BJU Int. 2014 Oct;114(4):568-75. doi: 10.1111/bju.12733. BJU Int. 2014. PMID: 24612148 Clinical Trial.
-
Efficacy and Safety of a Fixed-Dose Combination Therapy of Tamsulosin and Tadalafil for Patients With Lower Urinary Tract Symptoms and Erectile Dysfunction: Results of a Randomized, Double-Blinded, Active-Controlled Trial.J Sex Med. 2017 Aug;14(8):1018-1027. doi: 10.1016/j.jsxm.2017.06.006. J Sex Med. 2017. PMID: 28760246 Clinical Trial.
-
Efficacy and Safety of Hexanic Lipidosterolic Extract of Serenoa repens (Permixon) in the Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: Systematic Review and Meta-analysis of Randomized Controlled Trials.Eur Urol Focus. 2016 Dec;2(5):553-561. doi: 10.1016/j.euf.2016.04.002. Epub 2016 Apr 23. Eur Urol Focus. 2016. PMID: 28723522 Review.
-
The use of alpha1-adrenoceptor antagonists in lower urinary tract symptoms: beyond benign prostatic hyperplasia.Urology. 2003 Sep;62(3 Suppl 1):34-41. doi: 10.1016/s0090-4295(03)00472-2. Urology. 2003. PMID: 12957198 Review.
Cited by
-
Janus kinase inhibitors in palmoplantar pustulosis: a mixed-methods feasibility (JAKPPPOT) trial protocol.BMJ Open. 2025 Aug 21;15(8):e106361. doi: 10.1136/bmjopen-2025-106361. BMJ Open. 2025. PMID: 40840981 Free PMC article.
-
N-of-1 trials to facilitate evidence-based deprescribing: Rationale and case study.Br J Clin Pharmacol. 2022 Oct;88(10):4460-4473. doi: 10.1111/bcp.15442. Epub 2022 Jul 13. Br J Clin Pharmacol. 2022. PMID: 35705532 Free PMC article. Review.
-
Tracking Lower Urinary Tract Symptoms and Tamsulosin Side Effects Among Older Men Using a Mobile App (PERSONAL): Feasibility and Usability Study.JMIR Form Res. 2021 Dec 10;5(12):e30762. doi: 10.2196/30762. JMIR Form Res. 2021. PMID: 34889745 Free PMC article.
-
Perceptions of Older Men Using a Mobile Health App to Monitor Lower Urinary Tract Symptoms and Tamsulosin Side Effects: Mixed Methods Study.JMIR Hum Factors. 2021 Dec 24;8(4):e30767. doi: 10.2196/30767. JMIR Hum Factors. 2021. PMID: 34951599 Free PMC article.

