Adaptive Physics-Based Non-Rigid Registration for Immersive Image-Guided Neuronavigation Systems
- PMID: 34713074
- PMCID: PMC8521897
- DOI: 10.3389/fdgth.2020.613608
Adaptive Physics-Based Non-Rigid Registration for Immersive Image-Guided Neuronavigation Systems
Abstract
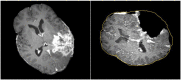
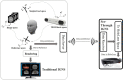
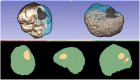
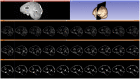
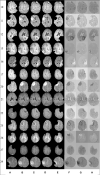
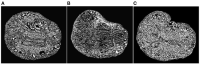
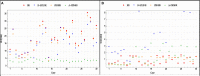
Objective: In image-guided neurosurgery, co-registered preoperative anatomical, functional, and diffusion tensor imaging can be used to facilitate a safe resection of brain tumors in eloquent areas of the brain. However, the brain deforms during surgery, particularly in the presence of tumor resection. Non-Rigid Registration (NRR) of the preoperative image data can be used to create a registered image that captures the deformation in the intraoperative image while maintaining the quality of the preoperative image. Using clinical data, this paper reports the results of a comparison of the accuracy and performance among several non-rigid registration methods for handling brain deformation. A new adaptive method that automatically removes mesh elements in the area of the resected tumor, thereby handling deformation in the presence of resection is presented. To improve the user experience, we also present a new way of using mixed reality with ultrasound, MRI, and CT. Materials and methods: This study focuses on 30 glioma surgeries performed at two different hospitals, many of which involved the resection of significant tumor volumes. An Adaptive Physics-Based Non-Rigid Registration method (A-PBNRR) registers preoperative and intraoperative MRI for each patient. The results are compared with three other readily available registration methods: a rigid registration implemented in 3D Slicer v4.4.0; a B-Spline non-rigid registration implemented in 3D Slicer v4.4.0; and PBNRR implemented in ITKv4.7.0, upon which A-PBNRR was based. Three measures were employed to facilitate a comprehensive evaluation of the registration accuracy: (i) visual assessment, (ii) a Hausdorff Distance-based metric, and (iii) a landmark-based approach using anatomical points identified by a neurosurgeon. Results: The A-PBNRR using multi-tissue mesh adaptation improved the accuracy of deformable registration by more than five times compared to rigid and traditional physics based non-rigid registration, and four times compared to B-Spline interpolation methods which are part of ITK and 3D Slicer. Performance analysis showed that A-PBNRR could be applied, on average, in <2 min, achieving desirable speed for use in a clinical setting. Conclusions: The A-PBNRR method performed significantly better than other readily available registration methods at modeling deformation in the presence of resection. Both the registration accuracy and performance proved sufficient to be of clinical value in the operating room. A-PBNRR, coupled with the mixed reality system, presents a powerful and affordable solution compared to current neuronavigation systems.
Keywords: deep learning; deformable registration; machine learning; medical image computing; mesh generation; mixed reality; neuronavigation systems; neurosurgery.
Copyright © 2021 Drakopoulos, Tsolakis, Angelopoulos, Liu, Yao, Kavazidi, Foroglou, Fedorov, Frisken, Kikinis, Golby and Chrisochoides.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
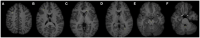
References
-
- Evren G, Chang E, Lamborn K, Tihan T, Chang C, Chang S, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. (2006) 105:34–40. 10.3171/jns.2006.105.1.34 - DOI - PubMed
-
- McGirt M, Mukherjee D, Chaichana K, Than K, Weingart J, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. (2009) 65:463–9; discussion: 469–70. 10.1227/01.NEU.0000349763.42238.E9 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

