An Improved CRISPR/dCas9 Interference Tool for Neuronal Gene Suppression
- PMID: 34713218
- PMCID: PMC8525373
- DOI: 10.3389/fgeed.2020.00009
An Improved CRISPR/dCas9 Interference Tool for Neuronal Gene Suppression
Abstract
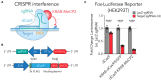
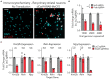
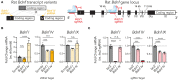
The expression of genetic material governs brain development, differentiation, and function, and targeted manipulation of gene expression is required to understand contributions of gene function to health and disease states. Although recent improvements in CRISPR/dCas9 interference (CRISPRi) technology have enabled targeted transcriptional repression at selected genomic sites, integrating these techniques for use in non-dividing neuronal systems remains challenging. Previously, we optimized a dual lentivirus expression system to express CRISPR-based activation machinery in post-mitotic neurons. Here we used a similar strategy to adapt an improved dCas9-KRAB-MeCP2 repression system for robust transcriptional inhibition in neurons. We find that lentiviral delivery of a dCas9-KRAB-MeCP2 construct driven by the neuron-selective human synapsin promoter enabled transgene expression in primary rat neurons. Next, we demonstrate transcriptional repression using CRISPR sgRNAs targeting diverse gene promoters, and show superiority of this system in neurons compared to existing RNA interference methods for robust transcript specific manipulation at the complex Brain-derived neurotrophic factor (Bdnf) gene. Our findings advance this improved CRISPRi technology for use in neuronal systems for the first time, potentially enabling improved ability to manipulate gene expression states in the nervous system.
Keywords: CRISPRi; KRAB-MeCP2; dCas9; gene regulation; neurons; transcription.
Copyright © 2020 Duke, Bach, Revanna, Sultan, Southern, Davis, Carullo, Bauman, Phillips and Day.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

