Polyunsaturated fatty acids cause physiological and behavioral changes in Vibrio alginolyticus and Vibrio fischeri
- PMID: 34713610
- PMCID: PMC8494716
- DOI: 10.1002/mbo3.1237
Polyunsaturated fatty acids cause physiological and behavioral changes in Vibrio alginolyticus and Vibrio fischeri
Abstract
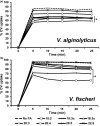
Vibrio alginolyticus and Vibrio (Aliivibrio) fischeri are Gram-negative bacteria found globally in marine environments. During the past decade, studies have shown that certain Gram-negative bacteria, including Vibrio species (cholerae, parahaemolyticus, and vulnificus) are capable of using exogenous polyunsaturated fatty acids (PUFAs) to modify the phospholipids of their membrane. Moreover, exposure to exogenous PUFAs has been shown to affect certain phenotypes that are important factors of virulence. The purpose of this study was to investigate whether V. alginolyticus and V. fischeri are capable of responding to exogenous PUFAs by remodeling their membrane phospholipids and/or altering behaviors associated with virulence. Thin-layer chromatography (TLC) analyses and ultra-performance liquid chromatography-electrospray ionization mass spectrometry (UPLC/ESI-MS) confirmed incorporation of all PUFAs into membrane phosphatidylglycerol and phosphatidylethanolamine. Several growth phenotypes were identified when individual fatty acids were supplied in minimal media and as sole carbon sources. Interestingly, several PUFAs acids inhibited growth of V. fischeri. Significant alterations to membrane permeability were observed depending on fatty acid supplemented. Strikingly, arachidonic acid (20:4) reduced membrane permeability by approximately 35% in both V. alginolyticus and V. fischeri. Biofilm assays indicated that fatty acid influence was dependent on media composition and temperature. All fatty acids caused decreased swimming motility in V. alginolyticus, while only linoleic acid (18:2) significantly increased swimming motility in V. fischeri. In summary, exogenous fatty acids cause a variety of changes in V. alginolyticus and V. fischeri, thus adding these bacteria to a growing list of Gram-negatives that exhibit versatility in fatty acid utilization and highlighting the potential for environmental PUFAs to influence phenotypes associated with planktonic, beneficial, and pathogenic associations.
Keywords: Aliivibrio fischeri; Vibrio; Vibrio alginolyticus; biofilm; fatty acids; motility; phospholipids.
© 2021 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures








References
-
- Adin, D. M. , Phillips, N. J. , Gibson, B. W. , Apicella, M. A. , Ruby, E. G. , McFall‐Ngai, M. J. , Hall, D. B. , & Stabb, E. V. (2007). Characterization of htrB and msbB mutants of the light organ symbiont Vibrio fischeri . Applied and Environmental Microbiology, 74, 633–644. 10.1128/AEM.02138-07. - DOI - PMC - PubMed
-
- Ahmed, R. , Rafiquzaman, S. M. , Hossain, M. T. , Lee, J. M. , & Kong, I. S. (2015). Species‐specific detection of Vibrio alginolyticus in shellfish and shrimp by real‐time PCR using the groEL gene . Aquaculture International, 24, 157–170. 10.1007/s10499-015-9916-5. - DOI
-
- Baker, L. Y. , Hobby, C. R. , Siv, A. W. , Bible, W. C. , Glennon, M. S. , Anderson, D. M. , Symes, S. J. , & Giles, D. K. (2018). Pseudomonas aeruginosa responds to exogenous polyunsaturated fatty acids (PUFAs) by modifying phospholipid composition, membrane permeability, and phenotypes associated with virulence. BMC Microbiology, 18, 117. 10.1186/s12866-018-1259-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

