Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy
- PMID: 34713636
- PMCID: PMC8696219
- DOI: 10.1002/cac2.12224
Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy
Abstract
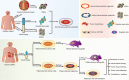
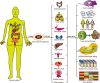
Patient-derived cancer cells (PDCs) and patient-derived xenografts (PDXs) are often used as tumor models, but have many shortcomings. PDCs not only lack diversity in terms of cell type, spatial organization, and microenvironment but also have adverse effects in stem cell cultures, whereas PDX are expensive with a low transplantation success rate and require a long culture time. In recent years, advances in three-dimensional (3D) organoid culture technology have led to the development of novel physiological systems that model the tissues of origin more precisely than traditional culture methods. Patient-derived cancer organoids bridge the conventional gaps in PDC and PDX models and closely reflect the pathophysiological features of natural tumorigenesis and metastasis, and have led to new patient-specific drug screening techniques, development of individualized treatment regimens, and discovery of prognostic biomarkers and mechanisms of resistance. Synergistic combinations of cancer organoids with other technologies, for example, organ-on-a-chip, 3D bio-printing, and CRISPR-Cas9-mediated homology-independent organoid transgenesis, and with treatments, such as immunotherapy, have been useful in overcoming their limitations and led to the development of more suitable model systems that recapitulate the complex stroma of cancer, inter-organ and intra-organ communications, and potentially multiorgan metastasis. In this review, we discuss various methods for the creation of organ-specific cancer organoids and summarize organ-specific advances and applications, synergistic technologies, and treatments as well as current limitations and future prospects for cancer organoids. Further advances will bring this novel 3D organoid culture technique closer to clinical practice in the future.
Keywords: 3D bio-printing; cancer organoids; cancer stroma; drug screening; organ-on-a-chip; personalized medicine; prognostic biomarker; tumor microenvironment.
© 2021 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Figures

References
-
- Invrea F, Rovito R, Torchiaro E, Petti C, Isella C, Medico E. Patient‐derived xenografts (PDXs) as model systems for human cancer. Curr Opin Biotechnol. 2020;63:151‐156. - PubMed
-
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. - PubMed
-
- Yan Q, Dong H, Su J, Han J, Song B, Wei Q, et al. A Review of 3D Printing Technology for Medical Applications. Engineering. 2018;4(5):729‐742.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

