Implications of Myocardial Infarction on Management and Outcome in Cardiogenic Shock
- PMID: 34713704
- PMCID: PMC8751815
- DOI: 10.1161/JAHA.121.021570
Implications of Myocardial Infarction on Management and Outcome in Cardiogenic Shock
Abstract
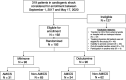
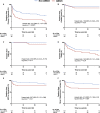
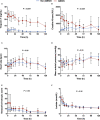
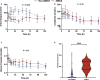
Background The randomized DOREMI (Dobutamine Compared to Milrinone) clinical trial evaluated the efficacy and safety of milrinone and dobutamine in patients with cardiogenic shock. Whether the results remain consistent when stratified by acute myocardial infarction remains unknown. In this substudy, we sought to evaluate differences in clinical management and outcomes of acute myocardial infarction complicated by cardiogenic shock (AMICS) versus non-AMICS. Methods and Results Patients in cardiogenic shock (n=192) were randomized 1:1 to dobutamine or milrinone. The primary composite end point in this subgroup analysis was all-cause in-hospital mortality, cardiac arrest, non-fatal myocardial infarction, cerebrovascular accident, the need for mechanical circulatory support, or initiation of renal replacement therapy (RRT) at 30-days. Outcomes were evaluated in patients with (n=65) and without (n=127) AMICS. The primary composite end point was significantly higher in AMICS versus non-AMICS (hazard ratio [HR], 2.21; 95% CI, 1.47-3.30; P=0.0001). The primary end point was driven by increased rates of all-cause mortality, mechanical circulatory support, and RRT. No differences in other secondary outcomes including cardiac arrest or cerebrovascular accident were observed. AMICS remained associated with the primary composite outcome, 30-day mortality, and RRT after adjustment for age, sex, procedural contrast use, multivessel disease, and inotrope type. Conclusions AMI was associated with increased rates of adverse clinical outcomes in cardiogenic shock along with increased rates of mortality and initiation of mechanical circulatory support and RRT. Contrast administration during revascularization likely contributes to increased rates of RRT. Heterogeneity of outcomes in AMICS versus non-AMICS highlights the need to study interventions in specific subgroups of cardiogenic shock. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT03207165.
Keywords: acute myocardial infarction; cardiogenic shock; inotrope; mechanical circulatory support; renal replacement therapy; revascularization.
Figures
References
-
- van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

