Mouse-INtraDuctal (MIND): an in vivo model for studying the underlying mechanisms of DCIS malignancy
- PMID: 34714554
- PMCID: PMC8738143
- DOI: 10.1002/path.5820
Mouse-INtraDuctal (MIND): an in vivo model for studying the underlying mechanisms of DCIS malignancy
Abstract
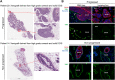
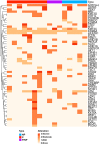
Due to widespread adoption of screening mammography, there has been a significant increase in new diagnoses of ductal carcinoma in situ (DCIS). However, DCIS prognosis remains unclear. To address this gap, we developed an in vivo model, Mouse-INtraDuctal (MIND), in which patient-derived DCIS epithelial cells are injected intraductally and allowed to progress naturally in mice. Similar to human DCIS, the cancer cells formed in situ lesions inside the mouse mammary ducts and mimicked all histologic subtypes including micropapillary, papillary, cribriform, solid, and comedo. Among 37 patient samples injected into 202 xenografts, at median duration of 9 months, 20 samples (54%) injected into 95 xenografts showed in vivo invasive progression, while 17 (46%) samples injected into 107 xenografts remained non-invasive. Among the 20 samples that showed invasive progression, nine samples injected into 54 xenografts exhibited a mixed pattern in which some xenografts showed invasive progression while others remained non-invasive. Among the clinically relevant biomarkers, only elevated progesterone receptor expression in patient DCIS and the extent of in vivo growth in xenografts predicted an invasive outcome. The Tempus XT assay was used on 16 patient DCIS formalin-fixed, paraffin-embedded sections including eight DCISs that showed invasive progression, five DCISs that remained non-invasive, and three DCISs that showed a mixed pattern in the xenografts. Analysis of the frequency of cancer-related pathogenic mutations among the groups showed no significant differences (KW: p > 0.05). There were also no differences in the frequency of high, moderate, or low severity mutations (KW; p > 0.05). These results suggest that genetic changes in the DCIS are not the primary driver for the development of invasive disease. © 2021 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: DCIS; DCIS model; Mouse-INtraDuctal (MIND); animal models; breast cancer; breast malignancy; ductal carcinoma in situ; nonmalignant breast cancers; precancer biology.
© 2021 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Figures




References
-
- Ozanne EM, Shieh Y, Barnes J, et al. Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat 2011; 129: 165–173. - PubMed
-
- Welch HG, Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med 1997; 127: 1023–1028. - PubMed
-
- Collins LC, Tamimi RM, Baer HJ, et al. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' Health Study. Cancer 2005; 103: 1778–1784. - PubMed
-
- Page DL, Dupont WD, Rogers LW, et al. Intraductal carcinoma of the breast: follow‐up after biopsy only. Cancer 1982; 49: 751–758. - PubMed
-
- Sanders ME, Schuyler PA, Dupont WD, et al. The natural history of low‐grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long‐term follow‐up. Cancer 2005; 103: 2481–2484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

