Modulating donor mitochondrial fusion/fission delivers immunoprotective effects in cardiac transplantation
- PMID: 34714588
- PMCID: PMC8813895
- DOI: 10.1111/ajt.16882
Modulating donor mitochondrial fusion/fission delivers immunoprotective effects in cardiac transplantation
Abstract
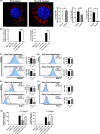
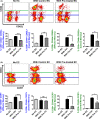
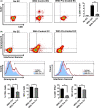
Early insults associated with cardiac transplantation increase the immunogenicity of donor microvascular endothelial cells (ECs), which interact with recipient alloreactive memory T cells and promote responses leading to allograft rejection. Thus, modulating EC immunogenicity could potentially alter T cell responses. Recent studies have shown modulating mitochondrial fusion/fission alters immune cell phenotype. Here, we assess whether modulating mitochondrial fusion/fission reduces EC immunogenicity and alters EC-T cell interactions. By knocking down DRP1, a mitochondrial fission protein, or by using the small molecules M1, a fusion promoter, and Mdivi1, a fission inhibitor, we demonstrate that promoting mitochondrial fusion reduced EC immunogenicity to allogeneic CD8+ T cells, shown by decreased T cell cytotoxic proteins, decreased EC VCAM-1, MHC-I expression, and increased PD-L1 expression. Co-cultured T cells also displayed decreased memory frequencies and Ki-67 proliferative index. For in vivo significance, we used a novel murine brain-dead donor transplant model. Balb/c hearts pretreated with M1/Mdivi1 after brain-death induction were heterotopically transplanted into C57BL/6 recipients. We demonstrate that, in line with our in vitro studies, M1/Mdivi1 pretreatment protected cardiac allografts from injury, decreased infiltrating T cell production of cytotoxic proteins, and prolonged allograft survival. Collectively, our data show promoting mitochondrial fusion in donor ECs mitigates recipient T cell responses and leads to significantly improved cardiac transplant survival.
Keywords: animal models: murine; basic (laboratory) research/science; heart transplantation/cardiology; immunobiology; immunosuppression/immune modulation; ischemia reperfusion injury (IRI); rejection: T cell mediated (TCMR); rejection: acute; translational research/science.
© 2021 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures





Comment in
-
Mitochondrial fission in allograft endothelial cells: A novel actionable target.Am J Transplant. 2022 Feb;22(2):337-338. doi: 10.1111/ajt.16911. Epub 2021 Dec 13. Am J Transplant. 2022. PMID: 34865296 No abstract available.
References
-
- Toyoda Y, Guy TS, Kashem A. Present status and future perspectives of heart transplantation. Circ J. 2013;77(5):1097‐1110. - PubMed
-
- Eisen HJ. Heart transplantation in adults: Diagnosis of acute allograft rejection. In: Dardas Todd F, ed. Waltham, MA: UpToDate; 2021.
-
- Libby P, Pober JS. Chronic rejection. Immunity. 2001;14(4):387‐397. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

