Salmonella Typhi Vi capsule prime-boost vaccination induces convergent and functional antibody responses
- PMID: 34714686
- PMCID: PMC9960181
- DOI: 10.1126/sciimmunol.abj1181
Salmonella Typhi Vi capsule prime-boost vaccination induces convergent and functional antibody responses
Abstract
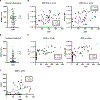
Vaccine development to prevent Salmonella Typhi infections has accelerated over the past decade, resulting in licensure of new vaccines, which use the Vi polysaccharide (Vi PS) of the bacterium conjugated to an unrelated carrier protein as the active component. Antibodies elicited by these vaccines are important for mediating protection against typhoid fever. However, the characteristics of protective and functional Vi antibodies are unknown. In this study, we investigated the human antibody repertoire, avidity maturation, epitope specificity, and function after immunization with a single dose of Vi-tetanus toxoid conjugate vaccine (Vi-TT) and after a booster with plain Vi PS (Vi-PS). The Vi-TT prime induced an IgG1-dominant response, whereas the Vi-TT prime followed by the Vi-PS boost induced IgG1 and IgG2 antibody production. B cells from recipients who received both prime and boost showed evidence of convergence, with shared V gene usage and CDR3 characteristics. The detected Vi antibodies showed heterogeneous avidity ranging from 10 μM to 500 pM, with no evidence of affinity maturation after the boost. Vi-specific antibodies mediated Fc effector functions, which correlated with antibody dissociation kinetics but not with association kinetics. We identified antibodies induced by prime and boost vaccines that recognized subdominant epitopes, indicated by binding to the de–O-acetylated Vi backbone. These antibodies also mediated Fc-dependent functions, such as complement deposition and monocyte phagocytosis. Defining strategies on how to broaden epitope targeting for S. Typhi Vi and enriching for antibody Fc functions that protect against typhoid fever will advance the design of high-efficacy Vi vaccines for protection across diverse populations.
Conflict of interest statement
Figures






References
-
- Käyhty H, Peltola H, Karanko V, Mäkelä PH, The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis 147, 1100 (1983). - PubMed
-
- Frasch CE, Borrow R, Donnelly J, Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 27 (Suppl. 2), B112–B116 (2009). - PubMed
-
- Maciejewski S, Ruckwardt TJ, Morabito KM, Foreman BM, Burgomaster KE, Gordon DN, Pelc RS, DeMaso CR, Ko SY, Fisher BE, Yang ES, Nair D, Foulds KE, Todd JP, Kong WP, Roy V, Aleshnick M, Speer SD, Bourne N, Barrett AD, Nason MC, Roederer M, Gaudinski MR, Chen GL, Dowd KA, Ledgerwood JE, Alter G, Mascola JR, Graham BS, Pierson TC, Distinct neutralizing antibody correlates of protection among related Zika virus vaccines identify a role for antibody quality. Sci. Transl. Med 12, eaaw9066 (2020). - PubMed
-
- Pittala S, Bagley K, Schwartz JA, Brown EP, Weiner JA, Prado IJ, Zhang W, Xu R, Ota-Setlik A, Pal R, Shen X, Beck C, Ferrari G, Lewis GK, LaBranche CC, Montefiori DC, Tomaras GD, Alter G, Roederer M, Fouts TR, Ackerman ME, Bailey-Kellogg C, Antibody Fab-Fc properties outperform titer in predictive models of SIV vaccine‐induced protection. Mol. Syst. Biol 15, e8747 (2019). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

