Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa
- PMID: 34714969
- PMCID: PMC9298689
- DOI: 10.1111/jth.15577
Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa
Abstract
Background: Factor XI (FXI) inhibition offers the promise of hemostasis-sparing anticoagulation for the prevention and treatment of thromboembolic events. Abelacimab (MAA868) is a novel fully human monoclonal antibody that targets the catalytic domain and has dual activity against the inactive zymogen Factor XI and the activated FXI.
Objectives: To investigate the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of single dose intravenous and multiple dose subcutaneous administration of abelacimab in healthy volunteers and patients with atrial fibrillation, respectively.
Patients/methods: In study ANT-003, healthy volunteers were administered single intravenous doses of abelacimab (30-150 mg) or placebo. The ANT-003 study also included a cohort of obese but otherwise healthy subjects. In study ANT-004, patients with atrial fibrillation were administered monthly subcutaneous doses of abelacimab (120 mg and 180 mg), or placebo, for 3 months. Key PK and PD parameters, including activated partial thromboplastin time (aPTT) and free FXI levels, as well as anti-drug antibodies (ADA) were assessed.
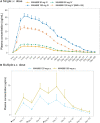
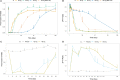
Results: Following intravenous administration of abelacimab, the terminal elimination half-life ranged from 25 to 30 days. One hour after the start of the intravenous infusion greater than 99% reductions in free FXI levels were observed. Following once monthly subcutaneous administration, marked reductions from baseline in free FXI levels were sustained. Parenteral administration of abelacimab demonstrated a favorable safety profile with no clinically relevant bleeding events.
Conclusions: Intravenous and multiple subcutaneous dose administration of abelacimab were safe and well tolerated. The safety, PK, and PD data from these studies support the clinical development of abelacimab.
Keywords: Factor XI; antibodies, monoclonal; blood; pharmacodynamics; pharmacokinetics.
© 2021 Anthos Therapeutics. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The author(s) declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: B. A. Yi, D. Freedholm, N. Widener, S. Coulter, and D. Bloomfield are employees or affiliates of Anthos Therapeutics. X. Wang, E. Simard, C. Cullen, N. M. Al‐Saady, N. E. Lepor, and M. Lovern declare no conflicts of interest.
Figures
References
-
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:3042‐3067. - PubMed
-
- Fredenburgh JC, Weitz JI. Factor XI as a target for new anticoagulants. Hamostaseologie. 2021;41:104‐110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

