Fibroblast heterogeneity in prostate carcinogenesis
- PMID: 34715252
- PMCID: PMC8788937
- DOI: 10.1016/j.canlet.2021.10.028
Fibroblast heterogeneity in prostate carcinogenesis
Abstract
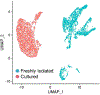
Our understanding of stromal components, specifically cancer-associated fibroblasts (CAF), in prostate cancer (PCa), has evolved from considering these cells as inert bystanders to acknowledging their significance as players in prostate tumorigenesis. CAF are multifaceted-they promote cancer cell growth, migration and remodel the tumor microenvironment. Although targeting CAF could be a promising strategy for PCa treatment, they incorporate a high but undefined degree of intrinsic cellular heterogeneity. The interaction between CAF subpopulations, with the normal and tumor epithelium and with other cell types is not yet characterized. Defining these interactions and the critical signaling nodes that support tumorigenesis will enable the development of novel strategies to control prostate cancer progression. Here we will discuss the origins, molecular and functional heterogeneity of CAF in PCa. We highlight the challenges associated with delineating CAF heterogeneity and discuss potential areas of research that would assist in expanding our knowledge of CAF and their role in PCa tumorigenesis.
Keywords: Cancer associated fibroblasts; Fibroblast heterogeneity; Myofibroblasts; Prostate cancer; Stromal heterogeneity.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interest.
Figures
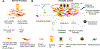

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

