Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review
- PMID: 34715347
- PMCID: PMC8548286
- DOI: 10.1016/j.cmi.2021.10.005
Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review
Abstract
Background: Vaccines are critical cost-effective tools to control the coronavirus disease 2019 (COVID-19) pandemic. However, the emergence of variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may threaten the global impact of mass vaccination campaigns.
Aims: The objective of this study was to provide an up-to-date comparative analysis of the characteristics, adverse events, efficacy, effectiveness and impact of the variants of concern for 19 COVID-19 vaccines.
Sources: References for this review were identified through searches of PubMed, Google Scholar, BioRxiv, MedRxiv, regulatory drug agencies and pharmaceutical companies' websites up to 22nd September 2021.
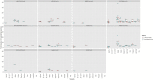
Content: Overall, all COVID-19 vaccines had a high efficacy against the original strain and the variants of concern, and were well tolerated. BNT162b2, mRNA-1273 and Sputnik V after two doses had the highest efficacy (>90%) in preventing symptomatic cases in phase III trials. mRNA vaccines, AZD1222, and CoronaVac were effective in preventing symptomatic COVID-19 and severe infections against Alpha, Beta, Gamma or Delta variants. Regarding observational real-life data, full immunization with mRNA vaccines and AZD1222 seems to effectively prevent SARS-CoV-2 infection against the original strain and Alpha and Beta variants but with reduced effectiveness against the Delta strain. A decline in infection protection was observed at 6 months for BNT162b2 and AZD1222. Serious adverse event rates were rare for mRNA vaccines-anaphylaxis 2.5-4.7 cases per million doses, myocarditis 3.5 cases per million doses-and were similarly rare for all other vaccines. Prices for the different vaccines varied from $2.15 to $29.75 per dose.
Implications: All vaccines appear to be safe and effective tools to prevent severe COVID-19, hospitalization, and death against all variants of concern, but the quality of evidence greatly varies depending on the vaccines considered. Questions remain regarding a booster dose and waning immunity, the duration of immunity, and heterologous vaccination. The benefits of COVID-19 vaccination outweigh the risks, despite rare serious adverse effects.
Keywords: COVID-19; Coronavirus; Delta; Efficacy; Review; SARS-CoV-2; Seroneutralization; Vaccines; Variants.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures




References
-
- World Health Organization Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... [Internet]. [cité 30 mai 2021]
-
- Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geidelberg L., et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021:1–17. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

