Abnormal tau in amyloid PET negative individuals
- PMID: 34715443
- PMCID: PMC9695003
- DOI: 10.1016/j.neurobiolaging.2021.09.019
Abnormal tau in amyloid PET negative individuals
Abstract
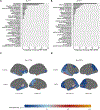
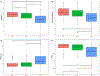
We examined the characteristics of individuals with biomarker evidence of tauopathy but without β-amyloid (Aβ) (A-T+) in relation to individuals with (A+T+) and without (A-T-) evidence of Alzheimer's disease (AD). We included 561 participants with Aβ and tau PET from the Alzheimer's Disease Neuroimaging Initiative (ADNI). We compared A-T- (n = 316), A-T+ (n = 63), and A+T+ (n = 182) individuals on demographics, amyloid, tau, hippocampal volumes, and cognition. A-T+ individuals were low on apolipoprotein E ɛ4 prevalence (17%) and had no evidence of subtly elevated brain Aβ within the negative range. The severity of tau deposition, hippocampal atrophy, and cognitive dysfunction in the A-T+ group was intermediate between A-T- and A+T+ (all p < 0.001). Tau uptake patterns in A-T+ individuals were heterogeneous, but approximately 29% showed tau deposition in the medial temporal lobe only, consistent with primary age-related tauopathy and an additional 32% showed a pattern consistent with AD. A-T+ individuals also share other features that are characteristic of AD such as cognitive impairment and neurodegeneration, but this group is heterogeneous and likely reflects more than one disorder.
Keywords: (18)F-flortaupir; Positron emission tomography; Primary age-related tauopathy; Tau; Tauopathies.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement
Dr. Yoon, Dr. Guo, Dr. Provost, Korman D, and Ward TJ report no disclosures relevant to the manuscript. Dr. Landau has served as a consultant to NeuroVison and Cortexyme. Dr. Jagust has served as a consultant to Genentech, Biogen, Novartis, CuraSen, Bioclinica, and Grifols.
Figures


References
-
- Altomare D, de Wilde A, Ossenkoppele R, Pelkmans W, Bouwman F, Groot C, van Maurik I, Zwan M, Yaqub M, Barkhof F, van Berckel BN, Teunissen CE, Frisoni GB, Scheltens P, van der Flier WM, 2019. Applying the ATN scheme in a memory clinic population. Neurology 93, e1635. - PubMed
-
- Bell WR, An Y, Kageyama Y, English C, Rudow GL, Pletnikova O, Thambisetty M, O’Brien R, Moghekar AR, Albert MS, Rabins PV, Resnick SM, Troncoso JC, 2019. Neuropathologic, genetic, and longitudinal cognitive profiles in primary age-related tauopathy (PART) and Alzheimer’s disease. Alzheimers Dement 15 (1), 8–16. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

