Principles for designing an optimal mRNA lipid nanoparticle vaccine
- PMID: 34715546
- PMCID: PMC8547895
- DOI: 10.1016/j.copbio.2021.09.016
Principles for designing an optimal mRNA lipid nanoparticle vaccine
Abstract
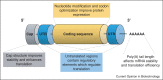
mRNA Lipid nanoparticles (LNPs) have recently been propelled onto the center stage of therapeutic platforms due to the success of the SARS-CoV-2 mRNA LNP vaccines (mRNA-1273 and BNT162b2), with billions of mRNA vaccine doses already shipped worldwide. While mRNA vaccines seem like an overnight success to some, they are in fact a result of decades of scientific research. The advantage of mRNA-LNP vaccines lies in the modularity of the platform and the rapid manufacturing capabilities. However, there is a multitude of choices to be made when designing an optimal mRNA-LNP vaccine regarding efficacy, stability and toxicity. Herein, we provide a brief on what we consider to be the most important aspects to cover when designing mRNA-LNPs from what is currently known and how to optimize them. Lastly, we give our perspective on which of these aspects is most crucial and what we believe are the next steps required to advance the field.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures


References
-
- Karikó K., Ni H., Capodici J., Lamphier M., Weissman D. mRNA is an endogenous ligand for toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. - PubMed
-
- Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

