Incorporating patient-centered quality-of-life measures for outcome assessment after Chiari malformation type I decompression in a pediatric population: a pilot study
- PMID: 34715646
- PMCID: PMC10193496
- DOI: 10.3171/2021.8.PEDS21228
Incorporating patient-centered quality-of-life measures for outcome assessment after Chiari malformation type I decompression in a pediatric population: a pilot study
Abstract
Objective: Optimal management of pediatric Chiari malformation type I (CM-I) is much debated, chiefly due to the lack of validated tools for outcome assessment, with very few tools incorporating patient-centered measures of health-related quality of life (HRQOL). Although posterior fossa decompression (PFD) benefits a subset of patients, prediction of its impact across patients is challenging. The primary aim of this study was to investigate the role of patient-centered HRQOL measures in the assessment and prediction of outcomes after PFD.
Methods: The authors collected HRQOL data from a cohort of 20 pediatric CM-I patients before and after PFD. The surveys included assessments of selected Patient-Reported Outcomes Measurement Information System (PROMIS) health domains and were used to generate the PROMIS preference (PROPr) score, which is a measure of HRQOL. PROMIS is a reliable standardized measure of HRQOL domains such as pain, fatigue, depression, and physical function, which are all relevant to CM-I. The authors then compared the PROPr scores with Chicago Chiari Outcome Scale (CCOS) scores derived from time-matched clinical documentation. Finally, the authors used the PROPr scores as an outcome measure to predict postsurgical HRQOL improvement at 1 year on the basis of patient demographic characteristics, comorbidities, and radiological and physical findings. The Wilcoxon signed-rank test, Mann-Whitney U-test, and Kendall's correlation were used for statistical analysis.
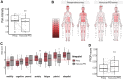
Results: Aggregate analysis revealed improvement of pain severity after PFD (p = 0.007) in anatomical patterns characteristic of CM-I. Most PROMIS domain scores trended toward improvement after surgery, with anxiety and pain interference reaching statistical significance (p < 0.002 and p < 0.03, respectively). PROPr scores also significantly improved after PFD (p < 0.008). Of the baseline patient characteristics, preexisting scoliosis was the most accurate negative predictor of HRQOL improvement after PFD (median -0.095 vs 0.106, p < 0.001). A correlation with modest magnitude (Kendall's tau range 0.19-0.47) was detected between the patient-centered measures and CCOS score.
Conclusions: The authors observed moderate improvement of HRQOL, when measured using a modified panel of PROMIS question banks, in this pilot cohort of pediatric CM-I patients after PFD. Further investigations are necessary to validate this tool for children with CM-I and to determine whether these scores correlate with clinical and radiographic findings.
Keywords: Chiari I malformation; health-related quality of life; outcome assessment; pediatric; posterior fossa decompression.
Conflict of interest statement
Research funding for this study was provided by a research grant from the American Syringomyelia & Chiari Alliance Project (ASAP).
Figures




References
-
- Haroun RI, Guarnieri M, Meadow JJ, Kraut M, Carson BS. Current opinions for the treatment of syringomyelia and Chiari malformations: survey of the Pediatric Section of the American Association of Neurological Surgeons. Pediatr Neurosurg. 2000;33(6):311–317. - PubMed
-
- Hersh DS, Groves ML, Boop FA. Management of Chiari malformations: opinions from different centers—a review. Childs Nerv Syst. 2019;35(10):1869–1873. - PubMed
-
- Rocque BG, George TM, Kestle J, Iskandar BJ. Treatment practices for Chiari malformation type I with syringomyelia: results of a survey of the American Society of Pediatric Neurosurgeons. J Neurosurg Pediatr. 2011;8(5):430–437. - PubMed
LinkOut - more resources
Full Text Sources

