Prediction of pathological response following neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: the PRE-PREVENCYS trial
- PMID: 34715822
- PMCID: PMC8556888
- DOI: 10.1186/s12885-021-08840-2
Prediction of pathological response following neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: the PRE-PREVENCYS trial
Abstract
Background: The recommended treatment for patients with non-metastatic muscle-invasive bladder cancer (MIBC) is neoadjuvant chemotherapy (NAC) and radical cystectomy (RC). Following NAC, 20-40% of patients experience a complete pathological response (pCR) in the RC specimen and these patients have excellent long-term overall survival. Subject to debate is, however, whether patients with a pCR to NAC benefit from RC, which is a major surgical procedure with substantial morbidity, and if these patients might be candidates for close surveillance instead. However, currently it is not possible to accurately identify patients with a pCR to NAC in whom RC might be withheld. The objective of this study is to assess whether pathological response in the RC specimen after NAC can be predicted based on clinical, radiological, and histological variables and on a wide set of molecular biomarkers assessed in tissue, blood and urine.
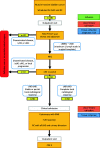
Methods: This is a multicentre, prospective cohort study, including patients with cT2a-T4a N0-N1 M0 urothelial cell MIBC who are scheduled to undergo cisplatin-based NAC followed by RC. Prior to start of therapy, a 2-Deoxy-2-[18F] fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is performed. Response to NAC is evaluated by CT-scan. Blood and urine, including cytology, are prospectively collected for biomarker analyses before and after NAC. Immediately before RC, participants undergo cystoscopy with bimanual examination and a re-staging transurethral resection (TUR) of all visible cancerous lesions or with biopsies from scar tissue. Subsequently, RC is performed in all patients. Tissue from the diagnostic TUR, the re-staging TUR, and the RC specimen is examined for the presence of urothelial cancer carcinoma and DNA and RNA is isolated for molecular analysis. The primary endpoint is the pathological stage (ypTN) in the RC and ePLND specimen and its association with clinical response.
Discussion: If the PRE-PREVENCYS trial shows that the absence of residual disease after NAC in patients with MIBC is accurately predicted, a randomized controlled trial is scheduled comparing the overall survival of NAC plus RC versus NAC followed by close surveillance for patients with a clinically complete response (PREVENCYS trial).
Trial registration: Netherlands Trial Register: NL8678; Registered 20 May 2020 https://www.trialregister.nl/trial/8678.
Keywords: Bladder cancer; Bladder-sparing; Cancer biomarkers; Cystectomy; Neoadjuvant chemotherapy; Residual tumour.
© 2021. The Author(s).
Conflict of interest statement
BJN is Associate Editor of BMC Cancer. All other authors declare that they have no competing interests.
Figures
References
-
- www.cijfersoverkanker.nl [updated 1-1-2020; cited 12-5-2021. Available from: https://iknl.nl/kankersoorten/blaaskanker/registratie/incidentie.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

