Mammalian sperm hyperactivation regulates navigation via physical boundaries and promotes pseudo-chemotaxis
- PMID: 34716265
- PMCID: PMC8612364
- DOI: 10.1073/pnas.2107500118
Mammalian sperm hyperactivation regulates navigation via physical boundaries and promotes pseudo-chemotaxis
Abstract
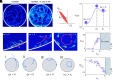
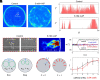
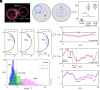
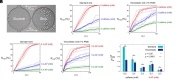
Mammalian sperm migration within the complex and dynamic environment of the female reproductive tract toward the fertilization site requires navigational mechanisms, through which sperm respond to the tract environment and maintain the appropriate swimming behavior. In the oviduct (fallopian tube), sperm undergo a process called "hyperactivation," which involves switching from a nearly symmetrical, low-amplitude, and flagellar beating pattern to an asymmetrical, high-amplitude beating pattern that is required for fertilization in vivo. Here, exploring bovine sperm motion in high-aspect ratio microfluidic reservoirs as well as theoretical and computational modeling, we demonstrate that sperm hyperactivation, in response to pharmacological agonists, modulates sperm-sidewall interactions and thus navigation via physical boundaries. Prior to hyperactivation, sperm remained swimming along the sidewalls of the reservoirs; however, once hyperactivation caused the intrinsic curvature of sperm to exceed a critical value, swimming along the sidewalls was reduced. We further studied the effect of noise in the intrinsic curvature near the critical value and found that these nonthermal fluctuations yielded an interesting "Run-Stop" motion on the sidewall. Finally, we observed that hyperactivation produced a "pseudo-chemotaxis" behavior, in that sperm stayed longer within microfluidic chambers containing higher concentrations of hyperactivation agonists.
Keywords: female reproductive tract; hyperactivation; mammalian sperm; navigation; sperm–wall interactions.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

