The lysosome as an imperative regulator of autophagy and cell death
- PMID: 34716768
- PMCID: PMC11071813
- DOI: 10.1007/s00018-021-03988-3
The lysosome as an imperative regulator of autophagy and cell death
Abstract
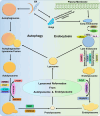
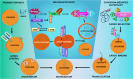
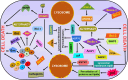
Lysosomes are single membrane-bound organelles containing acid hydrolases responsible for the degradation of cellular cargo and maintenance of cellular homeostasis. Lysosomes could originate from pre-existing endolysosomes or autolysosomes, acting as a critical juncture between autophagy and endocytosis. Stress that triggers lysosomal membrane permeabilization can be altered by ESCRT complexes; however, irreparable damage to the membrane results in the induction of a selective lysosomal degradation pathway, specifically lysophagy. Lysosomes play an indispensable role in different types of autophagy, including microautophagy, macroautophagy, and chaperone-mediated autophagy, and various cell death pathways such as lysosomal cell death, apoptotic cell death, and autophagic cell death. In this review, we discuss lysosomal reformation, maintenance, and degradation pathways following the involvement of the lysosome in autophagy and cell death, which are related to several pathophysiological conditions observed in humans.
Keywords: Autolysosome; Autophagic cell death; Autophagic lysosome reformation; Autophagy; Lysosome.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no conflict of interest with this manuscript.
Figures

References
-
- Mahapatra KK, et al. Molecular interplay of autophagy and endocytosis in human health and diseases. Biol Rev. 2019;94(4):1576–1590. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

