Efficacy and safety of standard of care with/without bevacizumab for platinum-resistant ovarian/fallopian tube/peritoneal cancer previously treated with bevacizumab: The Japanese Gynecologic Oncology Group study JGOG3023
- PMID: 34716979
- PMCID: PMC8748228
- DOI: 10.1111/cas.15185
Efficacy and safety of standard of care with/without bevacizumab for platinum-resistant ovarian/fallopian tube/peritoneal cancer previously treated with bevacizumab: The Japanese Gynecologic Oncology Group study JGOG3023
Abstract
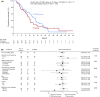
We investigated the efficacy and safety of further bevacizumab therapy in patients with platinum-resistant ovarian cancer whose disease had progressed after bevacizumab plus chemotherapy. In this multicenter, open-label, phase II trial (JGOG3023), patients were randomized 1:1 to a single-agent chemotherapy alone (either pegylated liposomal doxorubicin [40 or 50 mg/m2 administered intravenously], topotecan [1.25 mg/m2 intravenously], paclitaxel [80 mg/m2 intravenously], or gemcitabine [1000 mg/m2 intravenously]) or single-agent chemotherapy + bevacizumab (15 mg/m2 intravenously). The primary endpoint was investigator-assessed progression-free survival (PFS) according to RECIST version 1.1. Secondary endpoints were overall survival (OS), objective response rate (ORR), and response rate according to Gynecological Cancer Intergroup cancer antigen 125 criteria. In total, 103 patients were allocated to chemotherapy (n = 51) or chemotherapy + bevacizumab (n = 52). Median investigator-assessed PFS was 3.1 and 4.0 mo in each group, respectively (hazard ratio [HR] = 0.54, 95% confidence interval [CI]: 0.32-0.90, P = .0082). Median OS was 11.3 and 15.3 mo in each group, respectively (HR = 0.67, 95% CI: 0.38-1.17, P = .1556). Respective ORRs were 13.7% and 25.0% (P = .0599) and response rates were 16.7% and 21.4% (P = .8273). The incidence of grade ≥3 treatment-related AEs was 42.0% in the chemotherapy group and 54.9% in the chemotherapy + bevacizumab group; AEs were well tolerated, with only 2 and 12 events leading to discontinuation of therapy, respectively. Bevacizumab was effective beyond progressive disease and AEs were manageable. The observed improvement in PFS requires further verification.
Keywords: bevacizumab; fallopian tube cancer; ovarian cancer; peritoneal cancer; platinum resistance.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67:7‐30. - PubMed
-
- Pignata S, Lorusso D, Scambia G, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum‐resistant or platinum‐refractory advanced ovarian cancer (MITO 11): a randomised, open‐label, phase 2 trial. Lancet Oncol. 2015;16:561‐568. - PubMed
-
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2013;24 (Suppl 6):vi24‐vi32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

