The Structured Process to Identify Fit-For-Purpose Data: A Data Feasibility Assessment Framework
- PMID: 34716990
- PMCID: PMC9299818
- DOI: 10.1002/cpt.2466
The Structured Process to Identify Fit-For-Purpose Data: A Data Feasibility Assessment Framework
Abstract
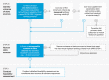
To complement real-world evidence (RWE) guidelines, the 2019 Structured Preapproval and Postapproval Comparative study design framework to generate valid and transparent real-world Evidence (SPACE) framework elucidated a process for designing valid and transparent real-world studies. As an extension to SPACE, here, we provide a structured framework for conducting feasibility assessments-a step-by-step guide to identify decision grade, fit-for-purpose data, which complements the United States Food and Drug Administration (FDA)'s framework for a RWE program. The process was informed by our collective experience conducting systematic feasibility assessments of existing data sources for pharmacoepidemiology studies to support regulatory decisions. Used with the SPACE framework, the Structured Process to Identify Fit-For-Purpose Data (SPIFD) provides a systematic process for conducting feasibility assessments to determine if a data source is fit for decision making, helping ensure justification and transparency throughout study development, from articulation of a specific and meaningful research question to identification of fit-for-purpose data and study design.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The following personal or financial relationships relevant to this manuscript existed during the conduct of the study: N.G., E.R., A.J., and P.M. are employees of and hold stock options or equity in Aetion Inc. R.R. is an employee of GlaxoSmithKline. U.C. and J.M. are employees of Pfizer Inc. N.G., E.R., U.C., J.M., and R.R. hold stock options in Pfizer Inc. The views expressed herein are the authors and not necessarily those of GlaxoSmithKline or Pfizer.
Figures




References
-
- HMA/EMA Joint Task Force on Big Data . Observational data (Real World Data) Subgroup report <www.ema.europa.eu/en/documents/report/observational‐data‐real‐world‐data...> (2019). Accessed June 16, 2021.
-
- CanREValue . Mapping Canadian Provincial Data Assets to Conduct Real‐World Studies on Cancer Drugs CanREValue Collaboration Data Working Group Interim Report 2020 <https://cc‐arcc.ca/wp‐content/uploads/2020/04/The‐CanREValue‐Data‐WG‐Int...>. Accessed June 15, 2021.
-
- Sherman, R.E. et al. Real‐world evidence — what is it and what can it tell us? N. Engl. J. Med. 375, 2293–2297 (2016). - PubMed
-
- U.S. Food & Drug Administration . Framework for FDA’s Real‐world Evidence Program <https://www.fda.gov/media/120060/download> (December 2018). Accessed June 15, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

