Central nervous system physiology
- PMID: 34717225
- PMCID: PMC8863401
- DOI: 10.1016/j.clinph.2021.09.013
Central nervous system physiology
Abstract
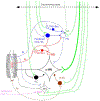
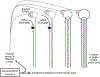
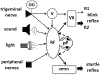
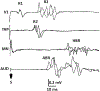
This is the second chapter of the series on the use of clinical neurophysiology for the study of movement disorders. It focusses on methods that can be used to probe neural circuits in brain and spinal cord. These include use of spinal and supraspinal reflexes to probe the integrity of transmission in specific pathways; transcranial methods of brain stimulation such as transcranial magnetic stimulation and transcranial direct current stimulation, which activate or modulate (respectively) the activity of populations of central neurones; EEG methods, both in conjunction with brain stimulation or with behavioural measures that record the activity of populations of central neurones; and pure behavioural measures that allow us to build conceptual models of motor control. The methods are discussed mainly in relation to work on healthy individuals. Later chapters will focus specifically on changes caused by pathology.
Keywords: Bereitschaftspotential; Computational motor control; Evoked potential; Reaction time; Transcranial direct current stimulation; Transcranial magnetic stimulation.
Copyright © 2021 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [U.Z. received grants from the German Ministry of Education and Research (BMBF), European Research Council (ERC), German Research Foundation (DFG), Janssen Pharmaceuticals NV and Takeda Pharmaceutical Company Ltd., and consulting fees from Bayer Vital GmbH, Pfizer GmbH and CorTec GmbH, all not related to this work. D.S received grants from NIH-R01-CRCNS-NS120579: Collaborative Research: Neural basis of motor expertise and NSF-M3X-1825942: Collaborative Research: Learning to control dynamically complex objects. None of the other authors report any Conflict of Interest.].
Figures












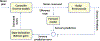

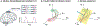
References
-
- Abdelmoula A, Baudry S, Duchateau J. Anodal transcranial direct current stimulation does not influence the neural adjustments associated with fatiguing contractions in a hand muscle. Eur J Appl Physiol 2019;119(3):597–609. - PubMed
-
- Aktekin B, Yaltkaya K, Ozkaynak S, Oguz Y. Recovery cycle of the blink reflex and exteroceptive suppression of temporalis muscle activity in migraine and tension-type headache. Headache 2001;41(2):142–9. - PubMed
-
- Albus J A theory of cerebellar function. Math Biosci 1971;10:25–61.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

