Advances and insights in the diagnosis of viral infections
- PMID: 34717656
- PMCID: PMC8556785
- DOI: 10.1186/s12951-021-01081-2
Advances and insights in the diagnosis of viral infections
Abstract
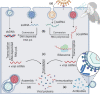
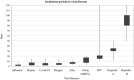
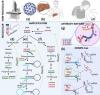
Viral infections are the most common among diseases that globally require around 60 percent of medical care. However, in the heat of the pandemic, there was a lack of medical equipment and inpatient facilities to provide all patients with viral infections. The detection of viral infections is possible in three general ways such as (i) direct virus detection, which is performed immediately 1-3 days after the infection, (ii) determination of antibodies against some virus proteins mainly observed during/after virus incubation period, (iii) detection of virus-induced disease when specific tissue changes in the organism. This review surveys some global pandemics from 1889 to 2020, virus types, which induced these pandemics, and symptoms of some viral diseases. Non-analytical methods such as radiology and microscopy also are overviewed. This review overlooks molecular analysis methods such as nucleic acid amplification, antibody-antigen complex determination, CRISPR-Cas system-based viral genome determination methods. Methods widely used in the certificated diagnostic laboratory for SARS-CoV-2, Influenza A, B, C, HIV, and other viruses during a viral pandemic are outlined. A comprehensive overview of molecular analytical methods has shown that the assay's sensitivity, accuracy, and suitability for virus detection depends on the choice of the number of regions in the viral open reading frame (ORF) genome sequence and the validity of the selected analytical method.
Keywords: Antibody-antigen complex; Biosensors; COVID-19; CRISPR-Cas for DNA-Sensors; Immunosensors; Photoluminescence; Polymerase chain reaction (PCR); SARS-CoV-2 virus detection; Surface plasmon resonance (SPR).
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Advancements in detection of SARS-CoV-2 infection for confronting COVID-19 pandemics.Lab Invest. 2022 Jan;102(1):4-13. doi: 10.1038/s41374-021-00663-w. Epub 2021 Sep 8. Lab Invest. 2022. PMID: 34497366 Free PMC article. Review.
-
A Recent Update on Advanced Molecular Diagnostic Techniques for COVID-19 Pandemic: An Overview.Front Immunol. 2021 Dec 14;12:732756. doi: 10.3389/fimmu.2021.732756. eCollection 2021. Front Immunol. 2021. PMID: 34970254 Free PMC article. Review.
-
Viral epidemiology and SARS-CoV-2 co-infections with other respiratory viruses during the first COVID-19 wave in Paris, France.Influenza Other Respir Viruses. 2021 Jul;15(4):425-428. doi: 10.1111/irv.12853. Epub 2021 Apr 4. Influenza Other Respir Viruses. 2021. PMID: 33817971 Free PMC article.
-
Detection of COVID-19: A review of the current literature and future perspectives.Biosens Bioelectron. 2020 Oct 15;166:112455. doi: 10.1016/j.bios.2020.112455. Epub 2020 Jul 21. Biosens Bioelectron. 2020. PMID: 32739797 Free PMC article. Review.
-
Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19.Biosens Bioelectron. 2020 Oct 15;166:112437. doi: 10.1016/j.bios.2020.112437. Epub 2020 Jul 15. Biosens Bioelectron. 2020. PMID: 32692666 Free PMC article.
Cited by
-
Systematically investigating the fluorescent signal readout of CRISPR-Cas12a for highly sensitive SARS-CoV-2 detection.Sens Actuators B Chem. 2022 Dec 15;373:132746. doi: 10.1016/j.snb.2022.132746. Epub 2022 Sep 30. Sens Actuators B Chem. 2022. PMID: 36212739 Free PMC article.
-
Recent trends and advancements in electrochemiluminescence biosensors for human virus detection.Trends Analyt Chem. 2022 Dec;157:116727. doi: 10.1016/j.trac.2022.116727. Epub 2022 Jul 5. Trends Analyt Chem. 2022. PMID: 35815064 Free PMC article. Review.
-
Magnet-assisted electrochemical immunosensor based on surface-clean Pd-Au nanosheets for sensitive detection of SARS-CoV-2 spike protein.Electrochim Acta. 2022 Feb 1;404:139766. doi: 10.1016/j.electacta.2021.139766. Epub 2021 Dec 23. Electrochim Acta. 2022. PMID: 34961798 Free PMC article.
-
Specific intracellular signature of SARS-CoV-2 infection using confocal Raman microscopy.Commun Chem. 2022;5(1):85. doi: 10.1038/s42004-022-00702-7. Epub 2022 Jul 25. Commun Chem. 2022. PMID: 35911504 Free PMC article.
-
Molecularly Imprinted Polymer-Based Sensors for SARS-CoV-2: Where Are We Now?Biomimetics (Basel). 2022 May 6;7(2):58. doi: 10.3390/biomimetics7020058. Biomimetics (Basel). 2022. PMID: 35645185 Free PMC article. Review.
References
-
- Valleron AJ, Meurisse S, Boelle PY. Historical Analysis of the 1889–1890 Pandemic in Europe. Int J Infect Dis. 2008;12:e95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

