Concurrent ibrutinib plus venetoclax in relapsed/refractory mantle cell lymphoma: the safety run-in of the phase 3 SYMPATICO study
- PMID: 34717692
- PMCID: PMC8556975
- DOI: 10.1186/s13045-021-01188-x
Concurrent ibrutinib plus venetoclax in relapsed/refractory mantle cell lymphoma: the safety run-in of the phase 3 SYMPATICO study
Abstract
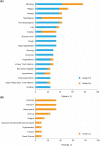
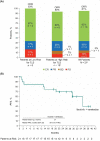
Ibrutinib plus venetoclax, given with an ibrutinib lead-in, has shown encouraging clinical activity in early phase studies in mantle cell lymphoma (MCL). The ongoing phase 3 SYMPATICO study evaluates the safety and efficacy of concurrently administered, once-daily, all-oral ibrutinib plus venetoclax in patients with relapsed/refractory MCL. A safety run-in (SRI) cohort was conducted to inform whether an ibrutinib lead-in should be implemented for the randomized portion. Patients received concurrent ibrutinib 560 mg continuously plus venetoclax in a 5-week ramp-up to venetoclax 400 mg for up to 2 years. The primary endpoint was occurrence of tumor lysis syndrome (TLS) and dose-limiting toxicities (DLTs). The SRI cohort enrolled 21 patients; six and 15 were in low- or increased-risk categories for TLS, respectively. During the 5-week venetoclax ramp-up, three patients had DLTs, and one patient at increased risk for TLS had a laboratory TLS; no additional TLS events occurred during follow-up. With a median follow-up of 31 months, the overall response rate was 81% (17/21); 62% (13/21) of patients had a complete response. SRI data informed that the randomized portion should proceed with concurrent ibrutinib plus venetoclax, with no ibrutinib lead-in. Ibrutinib plus venetoclax demonstrated promising efficacy; no new safety signals were observed.Trial registration: ClinicalTrials.gov, NCT03112174. Registered 13 April 2017, https://clinicaltrials.gov/ct2/show/NCT03112174 .
Keywords: Hematological cancers/lymphomas; Ibrutinib; Safety; Small molecule agents/kinase inhibitors; Venetoclax.
© 2021. The Author(s).
Conflict of interest statement
MW: honoraria from Janssen, AstraZeneca, OMI, Targeted Oncology, OncLive, Dava Oncology, Beijing Medical Award Foundation, Lu Daopei Medical Group, and Pharmacyclics LLC, an AbbVie Company; consulting or advisory role with Celgene, Janssen, AstraZeneca, MORE Health, Pulse Biosciences, Nobel Insights, Guidepoint Global, Kite Pharma, Juno Therapeutics, Loxo Oncology, InnoCare, Oncternal, and Pharmacyclics LLC, an AbbVie Company; research funding from Janssen, AstraZeneca, Acerta Pharma, Kite Pharma, Juno Therapeutics, Celgene, Loxo Oncology, VelosBio, Verastem, Molecular Templates, BioInvent, Oncternal, and Pharmacyclics LLC, an AbbVie Company; and travel accommodations from Janssen, Celgene, Juno Therapeutics, Kite Pharma, Loxo Oncology, VelosBio, Verastem, Molecular Templates, BioInvent, Oncternal, AstraZeneca, Acerta Pharma, and Pharmacyclics LLC, an AbbVie Company. RR: consulting or advisory role with Pharmacyclics LLC, an AbbVie Company; and research funding from Janssen and Pharmacyclics LLC, an AbbVie Company. RC: nothing to disclose. LK: employment with Aguettant; honoraria from Amgen, AbbVie, Celgene, Janssen, Takeda, and Sanofi; consulting or advisory role with Amgen, Celgene, Janssen, and Takeda; and travel accommodations from Amgen, Janssen, and Takeda. GC: consulting or advisory role with Bristol Myers Squibb; research funding from Merck Serono, Bristol Myers Squibb, Hutchison MediPharma, Regeneron, Isofol, AstraZeneca, Servier, and Pharmacyclics LLC, an AbbVie Company. WJ: consulting or advisory role with Sandoz Novartis, BeiGene, Janssen, Acerta, AstraZeneca, Loxo, and Epizyme; research funding from AbbVie, Acerta, Bayer, BeiGene, Janssen, MEI Pharma, Takeda, Telios, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company. KLW: consulting or advisory role with Amgen, Janssen, and AbbVie. MB: honoraria from Teva Pharma, Celltrion, and Roche Pharmaceuticals; consulting or advisory role with Celltrion, and Roche Pharmaceuticals; travel accommodations from Celltrion, Roche Pharmaceuticals, Takeda, and Gilead. GPC: honoraria from Roche, Takeda, Gilead, Pfizer, Bristol Myers Squibb, Merck Sharp & Dohme, Celleron, ADC Therapeutics, and Novartis; consulting or advisory role with Roche, Takeda, Gilead, Bristol Myers Squibb, Merck Sharp & Dohme, Celleron, and ADC Therapeutics; research funding from Bristol Myers Squibb, Celleron, Merck Sharp & Dohme, Celgene, and Amgen; speakers bureau with Roche, Takeda, Bristol Myers Squibb, Novartis, and Gilead; travel accommodations from Roche and Takeda. PE: nothing to disclose. FP: nothing to disclose. YL, KE, and JKN: employment with Pharmacyclics LLC, an AbbVie Company; and stock ownership in AbbVie. CST: honoraria from AbbVie, Janssen, Loxo, and BeiGene; consulting or advisory role with Janssen, Loxo, Roche, BeiGene, and AbbVie; and research funding from BeiGene, Janssen, and AbbVie.
Figures
References
-
- IMBRUVICA (ibrutinib) [prescribing information]. Sunnyvale: Pharmacyclics LLC; 2021.
-
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou Z, Cheng N, Fang B, McGreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA, Blum KA. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi: 10.1056/NEJMoa1306220. - DOI - PMC - PubMed
-
- Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho S-G, Bothos J, Goldberg JD, Enny C, Traina S, Balasubramanian S, Bandyopadhyay N, Sun S, Vermeulen J, Rizo A, Rule S. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. doi: 10.1016/S0140-6736(15)00667-4. - DOI - PubMed
-
- Rule S, Jurczak W, Jerkeman M, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho S-G, Thieblemont C, Zhou W, Henninger T, Goldberg J, Vermeulen J, Dreyling M. Ibrutinib versus temsirolimus: 3-year follow-up of patients with previously treated mantle cell lymphoma from the phase 3, international, randomized, open-label RAY study. Leukemia. 2018;32(8):1799–1803. doi: 10.1038/s41375-018-0023-2. - DOI - PMC - PubMed
-
- Rule S, Dreyling MH, Goy A, Hess G, Auer R, Kahl BS, Hernandez-Rivas JA, Qi K, Deshpande S, Parisi L, Wang ML. Long-term outcomes with ibrutinib versus the prior regimen: a pooled analysis in relapsed/refractory (R/R) mantle cell lymphoma (MCL) with up to 7.5 years of extended follow-up. Blood. 2019;134(Supplement_1):1538. doi: 10.1182/blood-2019-124691. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

