Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial
- PMID: 34717765
- PMCID: PMC8557559
- DOI: 10.1186/s13287-021-02631-z
Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial
Abstract
Objective: To evaluate the safety and efficacy of intra-articular (IA) injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE® for knee osteoarthritis.
Methods: This was a patient-blind, randomized, active-control trial consisted of 4 arms including hyaluronic acid (HA) control and 3 ELIXCYTE® doses. A total of 64 subjects were screened, and 57 subjects were randomized. The primary endpoints included the changes from baseline to post-treatment visit of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score at Week 24 and the incidence of adverse events (AEs) and serious adverse events (SAEs).
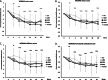
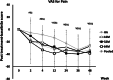
Results: No ELIXCYTE®-related serious adverse events were reported during 96 weeks of follow-up and no suspected unexpected serious adverse reaction (SUSAR) or death was reported. The changes of the primary endpoint, WOMAC pain score at Week 24, showed significant differences in all ELIXCYTE® groups, as well as in HA groups between post-treatment visit and baseline. The ELIXCYTE® groups revealed significant decreases at Week 4 compared to HA group in WOMAC total scores, stiffness scores, functional limitation scores suggested the potential of ELIXCYTE® in earlier onset compared to those from HA. The significant differences of visual analog scale (VAS) pain score and Knee Society Clinical Rating System (KSCRS) functional activities score at Week 48 after ELIXCYTE® administration suggested the potential of ELIXCYTE® in the longer duration of the effectiveness compared to HA group.
Conclusions: ELIXCYTE® for knee osteoarthritis treatment was effective, safe, and well-tolerated. The efficacy results were showed that ELIXCYTE® conferred the earlier onset of reductions in pain scores and improvements in functional scores than HA group.
Trial registration: ClinicalTrials.gov Identifier: NCT02784964. Registered 16 May, 2016-Retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT02784964.
Keywords: ADSCs; Adipose tissue-derived stem cells; ELIXCYTE®; HA; Hyaluronic acid; KSCRS; Knee osteoarthritis; VAS; WOMAC.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

