Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections
- PMID: 34717799
- PMCID: PMC8595755
- DOI: 10.1016/j.cell.2021.10.004
Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections
Abstract
The enteric nervous system (ENS) controls several intestinal functions including motility and nutrient handling, which can be disrupted by infection-induced neuropathies or neuronal cell death. We investigated possible tolerance mechanisms preventing neuronal loss and disruption in gut motility after pathogen exposure. We found that following enteric infections, muscularis macrophages (MMs) acquire a tissue-protective phenotype that prevents neuronal loss, dysmotility, and maintains energy balance during subsequent challenge with unrelated pathogens. Bacteria-induced neuroprotection relied on activation of gut-projecting sympathetic neurons and signaling via β2-adrenergic receptors (β2AR) on MMs. In contrast, helminth-mediated neuroprotection was dependent on T cells and systemic production of interleukin (IL)-4 and IL-13 by eosinophils, which induced arginase-expressing MMs that prevented neuronal loss from an unrelated infection located in a different intestinal region. Collectively, these data suggest that distinct enteric pathogens trigger a state of disease or tissue tolerance that preserves ENS number and functionality.
Keywords: enteric infections; enteric neurons; eosinophils; macrophages; neuroimmunology; small intestine.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
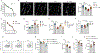
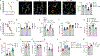
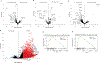
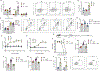



Comment in
-
Protecting your gut feelings: How intestinal infections keep things moving.Neuron. 2021 Nov 17;109(22):3545-3547. doi: 10.1016/j.neuron.2021.10.037. Neuron. 2021. PMID: 34793706
-
Protecting tissue integrity and enteric function: the case for type 2 inflammation and macrophages.Trends Parasitol. 2022 Mar;38(3):191-192. doi: 10.1016/j.pt.2021.12.008. Epub 2022 Jan 22. Trends Parasitol. 2022. PMID: 35078723 Free PMC article.
References
-
- Balemans D, Mondelaers SU, Cibert-Goton V, Stakenborg N, Aguilera-Lizarraga J, Dooley J, Liston A, Bulmer DC, Vanden Berghe P, Boeckxstaens GE, and Wouters MM (2017). Evidence for long-term sensitization of the bowel in patients with post-infectious-IBS. Scientific reports 7, 13606. 10.1038/s41598-017-12618-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

